Regulatory & Ethical Issues: Student Summary
- Describe the implications for veterinary drug law and pharmacy practice in food-producing animals and performance animals
- Describe what is the veterinarian-client-patient relationship
- Explore the categories and types of regulations of veterinary drugs
First of all, is there a difference in regulating veterinary drugs compared to human drugs?
Yes and No!
Yes! Humans are legally allowed to consume animal tissues and byproducts, except endangered species whereas food-producing animals (we will talk more about this in a bit) are consumed by humans that regulations should ensure the safety and effectiveness of the drugs to treat and to humans.
No! Both human and animal drugs are regulated by Food and Drug Administration (FDA) under Federal Food, Drug, and Cosmetic (FDC) Act.
Food-producing and performance animals?
These two categories of animals are important targets for veterinary drug law and pharmacy practice.
Food-producing animals
FDA considered major food-producing animals:
- Cows
- Pigs
- Chickens
- Turkeys
Regulations address the use of veterinary drugs in food-producing animals and the nature of the drug being administered.
As of November 2019, FDA has issued draft Guidance for “Compounding Animal Drugs from Bulk Drug Substances”. If finalized, this would allow drug compounding from bulk substances for food-producing animal patients. For now, It is not allowed.
What is a pharmacist’s job? Pharmacists should be aware of the intended use of the animal patient and follow all regulations and guidance when dispensing drugs to food-producing animal patients.
Performance animals
Two main types of performance animals:
- Racehorses
- Racing greyhounds
Association of Racing Commissioners International (ARCI) sets an international anti-doping standard.
What is a pharmacist’s job? Expected to be vigilant in preventing drug abuse by the caregivers for animal athletes.
Because of the unique situation of having non-human patients, there is the veterinarian-client-patient relationship.
| Requirements1 |
|
Let’s switch gears and talk about how veterinary drug laws have evolved.
Veterinary drug law
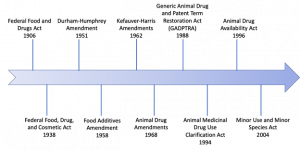
| Drug Law | Description |
| Federal Food and Drugs Act 1906 | Prohibited “misbranded” and “adulterated” foods and drugs in interstate commerce. |
| Federal Food, Drug, and Cosmetic Act 1932 | Required manufacturers to provide evidence that drug products were safe when used as directed, BEFORE the product would be approved for marketing. |
| Durham-Humphrey Amendment 1951 | Defined a category of drugs into Rx (prescribed drugs) and Over-The-Counter (OTC medications) |
| Food Additives Amendments 1958 | To gain FDA approval as an additive, manufacturers had to demonstrate that any drug residues present in the edible animal products from treated animals (i.e. meat, milk, eggs, honey) were safe for humans. |
| Kefauver-Harris Amendments 1962 | Required manufacturers to provide substantial evidence that drug products were effective for their intended use, in the form of adequate and well-controlled studies. |
| Animal Drug Amendments 1968 | Consolidated the principal provisions relating to approval of new drugs for administration to animals into a single section. “New animal drug” was defined as any drug intended for use in animals other than man, including any drug intended for use in animal feed. Both safety and efficacy profiles should be established for intended use to receive FDA approval. |
| Generic Animal Drug and Patent Term Restoration Act (GADPTRA) 1988 | Specified a category of drugs that could be dispensed only on the order of a licensed veterinarian. |
| Animal Medicinal Drug Use Clarification Act (AMDUCA) 1994 | Permits extra-label uses of certain approved new animal drugs and approved human drugs, by or on the lawful order of a veterinarian within the context of a Veterinarian-Client-Patient Relationship (VCPR). |
| Animal Drug Availability Act (ADAA) 1996 | Amended the definition of “substantial evidence of effectiveness” to make the drug review and approval process more flexible. Also, created a new category of drugs called veterinary feed directive (VFD) drugs. VFD drugs are intended for use in/on animal feed but are limited to use under professional supervision of a licensed veterinarian. |
| Minor Use and Minor Species (MUMS) Act 2004 | To provide alternate drug approval pathways that help pharmaceutical companies overcome financial hurdles associated with developing limited-demand animal drugs, similar to Orphan Drug Act for humans.
– Drugs used to treat uncommon diseases in the major animal species(horses, dogs, cats, cattle, pigs, chickens, and turkeys). – Drugs used to treat minor animal species(all animals species except humans that are not major species). |
Now we know the law, let’s take a look at what are the drugs that are under these regulations.
Categories of drugs used in animals
| Categories | Description |
| Prescription Drugs | Federal law restricts this drug to use by or on order of a licensed veterinarian. |
| Over-the-Counter Drugs | Drugs that could possibly be prepared with “adequate directions of use” where layperson can use the drug safely and effectively. Human OTC drugs are not labeled for use in any species other than humans unless specifically prescribed under order of a licensed veterinarian who has a valid VCPR with that animal. |
| Ethical Products | Drugs that are sold to veterinarians only as a condition of sale that is specified in a sales agreement or on the product label.2 |
| Compounded Preparations | Treated similarly to prescription drugs (Rx). Can only be prepared on the order of a licensed veterinarian with a VCPR in some of the states that require it. |
| Dietary Supplements and Nutraceuticals | Unlike equivalent human drugs, these are regulated by FDA as either foods or drugs. |
| Grooming Aids | A.K.A veterinary cosmetics such as shampoos, cream rinses, skin conditioners. These are not under the jurisdiction of FDA |
Food and Drug Administration (FDA)
- Governs the safety and efficacy of new animal drugs.
Drug Enforcement Administration (DEA)
- Prescriptions for controlled substances for veterinary patients are handled in the same way as those for human patients.
US Department of Agriculture (USDA)
- Responsible for regulating all veterinary biologics, including vaccines
- Unlike human medicine, FDA has no authority over the veterinary vaccine market.
**Pharmacists that are certified for administering immunization are only certified to immunize humans! The certification doesn’t extend to animal patients!
Environmental Protection Agency (EPA)
- Responsible for regulating manufacture, sale, and use of pesticides including rodenticides, insecticides, and germicidal preparations.
State Practice Acts
- It is important that the pharmacists know all laws and regulations regarding the practice of pharmacy in the states that they practice.
Reference
- The veterinarian-client-patient relationship (VCPR) | American Veterinary Medical Association. Accessed August 29, 2021. https://www.avma.org/resources-tools/pet-owners/petcare/veterinarian-client-patient-relationship-vcpr
- American Veterinary Medical Association. Principles of veterinary medical ethics of the AVMA. August 2019. https://www.avma.org/KB/Policies/Pages/Principles-of-Veterinary-Medical-Ethics-of-the-AVMA.aspx