Routes of Administration
Some routes of administration demand that products do not bring microbial contamination with them into the body. This is required because some routes of administration bypass the body’s natural defense mechanisms, or some tissues or organs are so sensitive and vital that such contamination could be serious.
All of these “sterility demanding” routes are parenteral routes.
NOTE: But not all parenteral routes are “sterility demanding” routes.
 The term parenteral means next to or beside the enteral. Enteral refers to the alimentary tract, so parenteral means sites that are outside of or beside the alimentary tract. Since oral, buccal, sublingual, and rectal comprise the enteral routes of administration, any other route is considered a parenteral administration site. Topical administration is a parenteral route that does not require sterile formulations.
The term parenteral means next to or beside the enteral. Enteral refers to the alimentary tract, so parenteral means sites that are outside of or beside the alimentary tract. Since oral, buccal, sublingual, and rectal comprise the enteral routes of administration, any other route is considered a parenteral administration site. Topical administration is a parenteral route that does not require sterile formulations.
The parenteral routes of administration are used for various reasons.
- If a drug is poorly absorbed when orally administered or is degraded by stomach acid or the gastrointestinal enzymes, then a parenteral route would be indicated.
- The parenteral routes are also preferred when a rapid and predictable drug response is desired as in an emergency situation.
- Parenteral routes of administration are also useful when a patient is uncooperative, unconscious, or unable to take drug via an enteral route.
- Parenteral routes are used when localized drug therapy is desired.
- They provide a predictable and nearly complete bioavailability.
But there are major disadvantages.
- Most of these parenteral formulations are more expensive than enteral route formulations.
- Most of these parenteral formulations must be sterile.
- Many formulations require that a skilled or trained person administer them.
- Once the drug is administered, it may be difficult to remove the dose if there is an adverse or toxic reaction.
These injection dependent routes have several characteristics in common. The formulations that can be used with injectables are limited to solutions, suspensions, and emulsions. Many of the routes have limited volumes of formulation that can be injected; excessive injection volumes will cause pain and cell necrosis.
Intravenous
 Several sites on the body are used to intravenously administer drugs: the veins of the antecubital area (in front of the elbow), the back of the hand, and some of the larger veins in the foot. On some occasions, a vein must be exposed by a surgical cut down.
Several sites on the body are used to intravenously administer drugs: the veins of the antecubital area (in front of the elbow), the back of the hand, and some of the larger veins in the foot. On some occasions, a vein must be exposed by a surgical cut down.
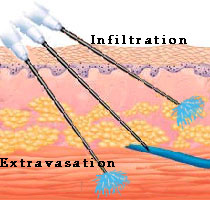
Intravenous administration will provide the most rapid onset of action of any parenteral route because there is no barrier to absorption. The drug is completely available to the body. Drugs that are too irritating for intramuscular or subcutaneous administration (e.g., chemotherapy agents) can be given by this route. Many different types of catheters (an indwelling soft tube) or needles are used to administer intravenous formulations. Placement of these devices is crucial to avoid problems of extravasation or infiltration.
There are several complications that can occur from intravenous administration.
- Thrombosis formation can result from many factors: extremes in solution pH, particulate material, irritant properties of the
 drug, needle or catheter trauma, and selection of too small a vein for the volume of solution injected.
drug, needle or catheter trauma, and selection of too small a vein for the volume of solution injected. - Phlebitis, or inflammation of the vein, can be caused by the same factors that cause thrombosis.
- Air emboli occur when air is introduced into the vein. The human body is not harmed by small amounts of air, but a good practice is to purge all air bubbles from the formulation and administration sets before use.
- Particulate material is generally small pieces of glass that chip from the formulation vial or rubber that comes from the rubber closure on injection vials. Although great care is taken to eliminate the presence of particulate material, a final filter in the administration line just before entering the venous system is a typical precaution.
Solutions are the most commonly administered intravenous formulation. They are usually aqueous but may also have glycols, alcohols, or other nonaqueous solvents in them. Injectable suspensions are difficult to formulate because they must possess syringeability and injectability. Syringeability refers to the ease at which the suspension can be withdrawn from a container into a syringe. It has been demonstrated that parenteral injections containing particles >25µm3 in size and having aspect ratios of >3.1 frequently cause syringe needle blockage. Injectability refers to the properties of the suspension while being injected, properties such as flow evenness, freedom from clogging, etc. Intravenously administered emulsions are heterogeneous formulations that contain both aqueous and non-aqueous components. Fat emulsions and total parenteral nutrition (TPN) emulsions are used to provide triglycerides, fatty acids, and calories for patients who cannot absorb them from the gastrointestinal tract.
Intramuscular
This route of administration is generally considered less hazardous and easier to use than the intravenous route. The onset of action is typically longer than with intravenous administration, but shorter than with subcutaneous administration. Patients generally experience more pain via intramuscular administration compared to intravenous administration.
generally experience more pain via intramuscular administration compared to intravenous administration.
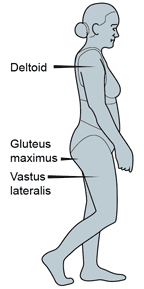 Intramuscular (IM) injections are made into the striated muscle fibers that are under the subcutaneous layer of the skin. Thus needles used for the injections are generally 1 inch to 1.5 inches long and are generally 19 to 22 gauge in size. The principal sites of injection are the gluteal (buttocks), deltoid (upper arm), and vastus lateralis (thigh) muscles. When administering intramuscular injections into the gluteus maximus, the size of the needle must be chosen based on the patient’s deposits of fat. If a needle used is too short to pass all the way through the fat into the muscle, then the injection will be made into the fat. Women tend to have more fat in this region than men, so the possibility of a intralipomatous injection is significant. It is estimated that few women and about 15% of men actually receive the intended intramuscular injection because an improper needle length was used.
Intramuscular (IM) injections are made into the striated muscle fibers that are under the subcutaneous layer of the skin. Thus needles used for the injections are generally 1 inch to 1.5 inches long and are generally 19 to 22 gauge in size. The principal sites of injection are the gluteal (buttocks), deltoid (upper arm), and vastus lateralis (thigh) muscles. When administering intramuscular injections into the gluteus maximus, the size of the needle must be chosen based on the patient’s deposits of fat. If a needle used is too short to pass all the way through the fat into the muscle, then the injection will be made into the fat. Women tend to have more fat in this region than men, so the possibility of a intralipomatous injection is significant. It is estimated that few women and about 15% of men actually receive the intended intramuscular injection because an improper needle length was used.
If a series of injections are to be given, the injection site is usually varied or rotated. Generally, only limited injection volumes can be given by intramuscular injection: 2 ml in the deltoid and thigh muscles, and up to 5 ml in the gluteus maximus.
The point of injection should be as far as possible from major nerves and blood vessels to avoid neural damage and accidental intravenous administration. To ensure that a blood vessel has not been entered, the syringe should be slightly aspirated after insertion and before injection to see if blood enters the syringe. Other injuries that can occur following intramuscular injection are abscesses, cysts, embolism, hematoma, skin sloughing, and scar formation.
The Z-tract injection technique is useful for medications that stain upper tissue such as iron dextran or drugs that irritate tissues such as diazepam. The skin is laterally displaced prior to injection, the needle is inserted, and the injection is performed. The needle then is withdrawn and the skin released. This creates a “Z” pattern that blocks infiltration of the drug into the subcutaneous tissue. These injections are generally 2-3 inches deep.
Intramuscular injections generally result in lower but more sustained blood concentrations than intravenous injections. Part of the reason is that intramuscular injections require an absorption step which delays the time to peak concentrations. When a formulation is injected, a “depot” forms inside the muscle tissue which acts as a repository for the drug. The absorption rate from this depot is dependent on many physiological factors such as muscle exercise, depth of injection, local blood flow supply, etc.
The absorption rate from the depot is also influenced by many formulation factors. Aqueous solutions typically provide the fastest absorption possible from the IM depot and oleaginous solutions provide slightly slower absorption. But to further alter the absorption rate, drugs are formulated as suspensions or colloids in aqueous and oleaginous solvents, or as oil-in-water emulsions and water-in-oil emulsions. Different salt forms of the drug may also be used to take advantage of a slower dissolution rate or a lower solubility inherent with the salt. All of these factors can be varied to achieve the desired absorption rate. For example, to achieve a very slow absorption rate, a low solubility salt form of the drug could be placed as a suspension in an oleaginous solvent. Here the salt would slowly dissolve due to its limited solubility, and then slowly diffuse through the oleaginous solvent.
Or another approach could be to make large particles of the drug (which will dissolve slowly) and put them in the oil phase of an oil-in-water emulsion. In this case, the drug would dissolve slowly, but would also diffuse slowly out of the oil phase, and would still need more time to diffuse through the water phase of the emulsion. Thus, there are three processes that influence the absorption rate of this type of formulation.
Intradermal
The dermis is the more vascular layer of the skin just beneath the epidermis. The usual site for 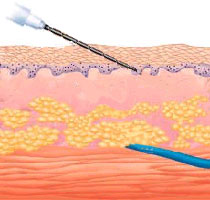 intradermal injections is the anterior surface of the forearm. Needles are generally 3/8 inches long and 25 to 26 gauge. Drugs that are intradermally injected are agents for diagnostic determinations, desensitization, or immunization. For this route of administration, 0.1 ml of solution is the maximum volume that can be administered. During injection a wheal, or raised blister-like area, forms on the skin surface as the solution is injected. The drug will be absorbed from this depot into the dermis.
intradermal injections is the anterior surface of the forearm. Needles are generally 3/8 inches long and 25 to 26 gauge. Drugs that are intradermally injected are agents for diagnostic determinations, desensitization, or immunization. For this route of administration, 0.1 ml of solution is the maximum volume that can be administered. During injection a wheal, or raised blister-like area, forms on the skin surface as the solution is injected. The drug will be absorbed from this depot into the dermis.
Subcutaneous
The subcutaneous (SC, SQ) route is one of the most versatile routes of administration in that it can be used for both short term and very long term therapies. The injection of a drug or the implantation of a device beneath the surface of the skin is made in the loose interstitial tissues of the upper arm, the anterior surface of the thigh, or the lower portion of the abdomen. The upper back can also be used as a site of subcutaneous administration. The site of injection is usually rotated when injections are frequently given. The maximum amount of medication that can be subcutaneously injected is about 2 ml. Needles are generally 3/8 to 1 inch in length and 24 to 27 gauge.
Absorption of drugs from the subcutaneous tissue is influenced by the same factors that determine the rate of absorption from intramuscular sites (slowly soluble salt forms, suspensions versus solutions, differences in particle size, viscosity of the injection vehicle, etc.); however, the vascularity in the subcutaneous tissue is less than that of muscle tissue, and therefore absorption may be slower than after intramuscular administration. But absorption after subcutaneous administration is generally more rapid and predictable than after oral administration. There are several ways to change the absorption rate:
- use heat or massage the site to increase the absorption rates of many drugs.
- co-administer vasodilators or hyaluronidase to increase absorption rates of some drugs. Conversely, epinephrine decreases blood flow which can decrease the absorption rate.
Many different solution and suspension formulations are given subcutaneously. Heparin, enoxaparin, and insulin are the most important drugs routinely administered by this route. Drugs that are administered by the subcutaneous route must be soluble and potent in small concentrations since only a very small volume can be injected.
In spite of the advantages of this route of administration, there are some precautions to observe.
- Drugs which are irritating or those in very viscous (thick) suspensions may produce induration, sloughing, or abscess formation and may be painful to the patient.
- Irritating drugs and vasoconstrictors can lead to abscesses or necrosis.
- If frequent injections are required, the injection sites must be rotated.
One of the most obvious ways to achieve very long term drug release is to place the drug in a delivery system or device that can be implanted into a body tissue. The subcutaneous tissue is the ideal site for implantation of such devices. Implantation often requires a surgical procedure or a specialized injection device. The fact that the device will be in constant contact with the  subcutaneous tissue requires that the device materials be biocompatible (e.g., not irritating) and do not promote infection or sterile abscess. Another advantage is that the device can be easily removed if necessary.
subcutaneous tissue requires that the device materials be biocompatible (e.g., not irritating) and do not promote infection or sterile abscess. Another advantage is that the device can be easily removed if necessary.
Examples of implantable devices are Norplant®, Oreton®, Percorten® and an osmotically driven mini-pump (Alzet®) which can deliver drug solutions for up to twenty-one days. Many devices provide drug release for months or years. ViadurTM DUROS® implants are made from a titanium alloy and are capable of delivering the drug for up to one year. Supprelin LA® implants are also able to provide drug release for up to one year. Implanon® and Nexplanon® release a drug for three years and are used as contraceptives. Other devices include degradable microspheres, vapor pressure devices for morphine release, osmotic pressure devices to deliver insulin, and magnetically or ultrasonically activated pellets.
Sometimes ports and pumps are placed in the subcutaneous space and the attached delivery catheter is placed in a vein, cavity, or artery.
Epidural
The brain and spinal cord are covered by three membranes called meninges. The dura mater is the outermost of these protective membranes. The spinal cord is protected from injury as it goes down the back by passing through the central cavities of the vertebrae in the vertebral column. The epidural space is the space in the central cavities between the dura mater (covering the spinal cord) and the vertebral column.
Epidural administration is an effective means of controlling and relieving chronic pain. It is commonly used in the labor and delivery settings, and in postoperative settings. Local anesthetics such as bupivacaine, chloroprocaine, or lidocaine are drugs that are often delivered through the epidural route. They are often given concomitantly with other pain medications, especially narcotics, to decrease the dose of opioids and provide multi-nodal pain relief. The advantage of this route of administration is that drug dosages are generally much lower than when given by other routes and typically produce fewer side effects. Studies have shown that epidural administration produces longer lasting pain relief, increased patient alertness, and earlier ambulation.
When epidural administration will be temporary or short-term, a catheter is inserted into the epidural space but is generally not sutured into place. The catheter exits from the insertion site on the back. If the administration will be permanent or long-term, the catheter is “tunneled” and exits on the side of the body or on the abdomen. A variation of the technique is to subcutaneously implant a Port-a-cath® and connect that device to the indwelling catheter.
It is obvious why sterile compounded formulations must be used via this route of administration. Preservatives are strictly contraindicated in epidural formulations to prevent nerve damage.
Intrathecal
The dura mater is the outermost meninge and the arachnoid mater is the innermost meninge. Intrathecal injections place formulations in the subarachnoid space, e.g., underneath the arachnoid mater. This space is filled with the cerebrospinal fluid that circulates around the spinal cord and the brain. Intrathecal injections allow dosages that may be about one-tenth those given by epidural administration. However, intrathecal administration carries a greater risk and consequence of bacterial contamination than epidural administration because the cerebrospinal fluid is a good medium for bacteria growth. These injections may also cause “spinal headaches.” Spinal headaches occur when the needle puncture does not seal off and cerebral spinal fluid continually leaks out into the epidural space.
Intrathecal administration is often used to give single injections of narcotics for postoperative pain management. Implantable infusion pumps are used to chronically administer medications to the intrathecal space. These administration techniques allow for very low doses of medications to be given in a controlled manner and reduces the incidence of side effects. The reservoirs in these pumps typically hold between 18 – 50 ml of solution; therefore, the solution concentrations will be high. Formulating high concentration solutions may entail problems with solubility, pH, buffering, etc. Also, these solutions must be preservative free to avoid nerve damage.
Injection independent routes do not require a needle or catheter for administration.
Many products and preparations administered by injection independent routes are required to be sterile. For example, ophthalmics, intranasal (metered) formulations, and inhalation preparations are sterile. However, formulations for some ointments, otic, and vaginal preparations may be sterile. Most are described elsewhere on the pharmlabs website and have been linked below.