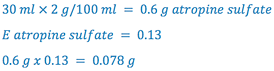Ophthalmic Preparations
Upon completion of this exercise, you should be able to:
- Define buffers, buffer capacity, isotonicity, iso-osmoticity, osmotic pressure, hypertonicity, hypotonicity.
- Describe the use buffers in pharmaceutical solutions.
- Identify the range of solution pH considered safe for ophthalmic solutions.
- Formulate and analyze a buffer solution of desired pH and buffer capacity.
- Explain the importance of isotonicity in ophthalmic solutions.
- Explain the importance of sterility in ophthalmic solutions.
- Explain the role of preservatives in pharmaceutical solutions.
- Formulate and prepare pharmaceutically and physiologically acceptable ophthalmic solutions.
Ophthalmic preparations deliver a drug on the eye, into the eye, or onto the conjunctiva. Drugs are administered to the eye for local effects such as miosis, mydriasis, and anesthesia, or to reduce intraocular pressure in treating glaucoma. Transcorneal transport (i.e., drug penetration into the eye) is not an effective process. It is estimated that only one-tenth of a dose penetrates into the eye.
Following ophthalmic administration, it is possible to get systemic effects. Drug can enter the systemic circulation by two ways:
- Drug that enters the lacrimal canalicula is emptied into the gastrointestinal tract
- Drug can be absorbed through the conjunctiva
Formulations used include aqueous solutions, aqueous suspensions, ointments, and inserts. Every ophthalmic product must be sterile in its final container to prevent microbial contamination of the eye. Preservatives are added to the formulation to maintain sterility once the container has been opened. Ophthalmic formulations also require that the pH, buffer capacity, viscosity, and tonicity of the formulation is carefully controlled.
 Most compounded ophthalmic solutions and suspensions are packaged in eye dropper bottles. Patients should be shown how to properly instill the drops in their eyes, and every effort should be made to emphasize the need for instilling only one drop per administration, not two or three.
Most compounded ophthalmic solutions and suspensions are packaged in eye dropper bottles. Patients should be shown how to properly instill the drops in their eyes, and every effort should be made to emphasize the need for instilling only one drop per administration, not two or three.
In an effort to maintain longer contract between the drug and the surrounding tissue, suspensions, ointments, and inserts have been developed. When aqueous suspensions are used, the particle size is kept to a minimum to prevent irritation of the eye. It is possible to find particles adhering to the conjunctiva after administration of this dosage form. Ointments tend to keep the drug in contact with the eye longer than suspensions. Most ophthalmic ointment bases are a mixture of mineral oil and white petrolatum and have a melting point close to body temperature. But ointments tend to blur patient vision as they remain viscous and are not removed easily by the tear fluid. Therefore, ointments are generally used at night as adjunctive therapy to eye drops which are used during the day. Ophthalmic ointment tubes are typically small holding approximately 3.5 g of ointment and fitted with narrow gauge tips which permit the extrusion of narrow bands of ointment.
There are three unique ways that drug can be lost following ophthalmic administration:
- Immediate loss due to spillage. The normal volume of tears in the eye is estimated to be 7 microliters, and if blinking occurs, the eye can hold up to 10 microliters without spillage. The normal commercial eye dropper dispenses 50 microliters of solution; thus, more than half of the dose will be lost from the eye by overflow. The ideal volume of drug solution to administer would be 5 to 10 microliters; however, there are no microliter dosing eye droppers generally available to patients.
- Lacrimal drainage. Tears wash the eyeball as they flow from the lacrimal gland across the eye and drain into the lacrimal canalicula. In man, the rate of tear production is approximately 2 microliters per minute; thus, the entire tear volume in the eye turns over every 2 to 3 minutes. This rapid washing and turnover also accounts for loss of an ophthalmic dose in a relatively short period of time.
- Drug absorption into the conjunctiva and its rapid removal from the ocular tissues by the peripheral blood flow.
 Ophthalmic solutions are sterile, free from foreign particles, and specially prepared for instillation in the eye. Most ophthalmic solutions are dispensed in eye dropper bottles. Patients should be shown how to properly instill the drops in their eyes, and every effort should be made to emphasize the need for instilling only one drop per administration, not two or three. When more than one drop is to be administered, wait at least five minutes between administrations. Immediately after instilling a drop on the eye, place pressure on the lacrimal sac for one or two minutes. This will reduce the rate of drug loss through this pathway.
Ophthalmic solutions are sterile, free from foreign particles, and specially prepared for instillation in the eye. Most ophthalmic solutions are dispensed in eye dropper bottles. Patients should be shown how to properly instill the drops in their eyes, and every effort should be made to emphasize the need for instilling only one drop per administration, not two or three. When more than one drop is to be administered, wait at least five minutes between administrations. Immediately after instilling a drop on the eye, place pressure on the lacrimal sac for one or two minutes. This will reduce the rate of drug loss through this pathway.
Ophthalmic suspensions are aqueous formulations that contain solid particles. The particle size must be kept to a minimum to prevent irritation of the eye. It has been recommended that particles be less than 10 microns in size to minimize irritation to the eye. The micronized form of the drug can be used to meet this requirement. There is a tendency of the solid undissolved particles to adhere to the conjunctiva. As drug is absorbed, these solid particles will dissolve to replenish the absorbed drug. This reservoir effect increases the contact time and duration of action of a suspension compared to a solution.
Administering Ophthalmic Dosage Forms
How to Use Ophthalmic Drops
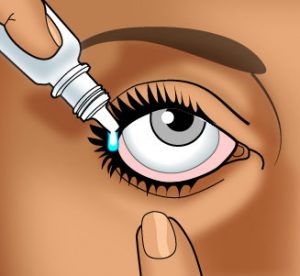 Wash your hands carefully with soap and warm water.
Wash your hands carefully with soap and warm water.- If the product container is transparent, check the solution before use. If it is discolored or has changed in any way since it was purchased (e.g., particles in the solution, color change), do not use the solution.
- If the product container has a depressible rubber bulb, draw up a small amount of medication into the eye dropper by first squeezing, then relieving pressure on the bulb.
- Tilt the head back with chin tilted up and look toward the ceiling.
- With both eyes open, gently draw down the lower lid of the affected eye with your index finger.
- In the “gutter” formed, place one drop of the solution.
IMPORTANT: The dropper or administration tip should be held as near as possible to the lid without actually touching the eye. DO NOT allow the dropper or administration tip to touch any surface. - If possible, hold the eyelid open and do not blink for 30 seconds.
- You may want to press your finger against the inner corner of your eye for one minute. This will keep the medication in your eye.
- Tightly cap the bottle.
Comments
- This is a sterile solution. Contamination of the dropper or eye solution can lead to a serious eye infection.
- If irritation persists or increases, discontinue use immediately.
- Generally, eye makeup should be avoided while using eye solutions.
- You may want to use a mirror when applying the drops, or it may be much easier to have someone help you instill your eye drops.
How To Administer An Ophthalmic Ointment
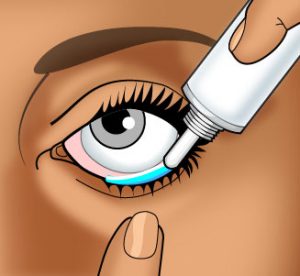 Wash your hands carefully with soap and warm water.
Wash your hands carefully with soap and warm water.- You may want to hold the ointment tube in your hand for a few minutes to warm and soften the ointment.
- Gently cleanse the affected eyelid with warm water and a soft cloth before applying the ointment.
- In front of a mirror, with the affected eye looking upward, gently pull the lower eyelid downward with your index finger to form a pouch.
- Squeeze a thin line (approximately ¼ – ½ inch) of the ointment along the pouch.
IMPORTANT: Be very careful when applying this ointment. DO NOT allow the tip of the ointment tube to touch the eyelid, the eyeball, your finger, or any surface. - Close the eye gently and rotate the eyeball to distribute the ointment. You may blink several times to evenly spread the ointment.
- Replace the cap on the ointment tube.
After you apply the ointment, your vision may be blurred temporarily. Do not be alarmed. This will clear up in a short while, but do not drive a car or operate machinery until your vision has cleared.
Comments
- This is a sterile ointment. Contamination of the tip or the cap of the tube can lead to a serious eye infection.
- If irritation persists or increases, discontinue use immediately.
- Generally, eye makeup should be avoided while using eye ointments.
- It may be much easier to have someone help you apply your eye ointment
In an effort to maintain longer contact between the drug and ocular tissue, ointments and inserts have been used.
Ophthalmic ointments tend to keep the drug in contact with the eye longer than suspensions. Most ophthalmic ointment bases are a mixture of mineral oil and white petrolatum and have a melting point close to body temperature. Sometimes anhydrous lanolin is used to take up an ingredient that was dissolved in a small amount of water to affect dissolution. The aqueous solution is incorporated into the lanolin and then the lanolin is mixed with the remaining ointment base ingredients.
![]() Ointments must be nonirritating and free from grittiness so the micronized form of the ingredients is required. Sterile ointments are prepared by first sterilizing all of the individual ingredients and then combining them under aseptic conditions. The prepared ointment is then packaged in a sterile container such as an ointment tube.
Ointments must be nonirritating and free from grittiness so the micronized form of the ingredients is required. Sterile ointments are prepared by first sterilizing all of the individual ingredients and then combining them under aseptic conditions. The prepared ointment is then packaged in a sterile container such as an ointment tube.
Most ointments tend to blur patient vision as they remain viscous and are not removed easily by the tear fluid. Thus ointments are generally used at night as adjunctive therapy to eye drops used during the day. Ophthalmic ointment tubes are typically small holding approximately 3.5 g of ointment and fitted with narrow gauge tips which permit the extrusion of narrow bands of ointment.
Ocular inserts are not compounded but must be manufactured. Ocusert is a nonerodible device designed to deliver pilocarpine for several days in the treatment of glaucoma. Some inserts are designed to dissolve in tear fluid. These inserts are made of dried polymeric solutions that have been fashioned into a film or rod. An example of this type of insert is Lacrisert used to treat moderate to severe dry eye syndrome. Inserts are placed in the cul-de-sac between the eyeball and the eyelid. The biggest disadvantage of inserts is their tendency to float on the eyeball, particularly in the morning upon arising.
Solution pH
The physiologic pH of blood and tears is approximately 7.4. Thus, from a comfort and safety standpoint, this would be the optimal pH of ophthalmic and parenteral solutions. This may not be possible, however, from a perspective of solubility, chemical stability or therapeutic activity. Thus, some compromise must be made and product stability must be considered paramount.
When a formulation is administered to the eye, it stimulates the flow of tears. Tear fluid is capable of quickly diluting and buffering small volumes of added substances, thus the eye can tolerate a fairly wide pH range. Ophthalmic solutions may range from pH 4.5 – 11.5. But the useful range to prevent corneal damage is 6.5 to 8.5.
Once we have determined the optimal pH of a product, we need a mechanism for adjusting and maintaining the pH of the solution.
Buffers and Buffer Capacity
Buffers are compounds that resist changes in pH upon the addition of limited amounts of acids or bases. Buffer systems are usually composed of a weak acid or base and its conjugate salt. The components act in such a way that addition of an acid or base results in the formulation of a salt causing only a small change in pH.
The pH of a buffer system is given by the Henderson-Hasselbach equation:
![]() (for a weak acid and its salt)
(for a weak acid and its salt)
![]() (for a weak base and its salt)
(for a weak base and its salt)
where [salt], [acid] and [base] are the molar concentrations of salt, acid and base.
Buffer capacity is a measure of the efficiency of a buffer in resisting changes in pH. Conventionally, the buffer capacity (![]() ) is expressed as the amount of strong acid or base, in gram-equivalents, that must be added to 1 liter of the solution to change its pH by one unit.
) is expressed as the amount of strong acid or base, in gram-equivalents, that must be added to 1 liter of the solution to change its pH by one unit.
Calculate the buffer capacity as:
![]()
![]() = gram equivalent of strong acid/base to change pH of 1 liter of buffer solution
= gram equivalent of strong acid/base to change pH of 1 liter of buffer solution
![]() = the pH change caused by the addition of strong acid/base
= the pH change caused by the addition of strong acid/base
In practice, smaller pH changes are measured and the buffer capacity is quantitatively expressed as the ratio of acid or base added to the change in pH produced (e.g., mEq./pH for x volume). The buffer capacity depends essentially on 2 factors:
- Ratio of the salt to the acid or base. The buffer capacity is optimal when the ratio is 1:1; that is, when pH = pKa
- Total buffer concentration. For example, it will take more acid or base to deplete a 0.5 M buffer than a 0.05 M buffer.
The relationship between buffer capacity and buffer concentrations is given by the Van Slyke equation:
![]()
where C = the total buffer concentration (i.e. the sum of the molar concentrations of acid and salt).
Just as we must often compromise the optimal pH for a product, so must we compromise on the optimal buffer capacity of our solution. On the one hand, buffer capacity must be large enough to maintain the product pH for a reasonably long shelf-life. Changes in product pH may result from interaction of solution components with one another or with the product package (glass, plastic, rubber closures, etc.). On the other hand, the buffer capacity of ophthalmic and parenteral products must be low enough to allow rapid readjustment of the product to physiologic pH upon administration. The pH, chemical nature, and volume of the solution to be administered must all be considered. Buffer capacities ranging from 0.01 – 0.1 are usually adequate for most pharmaceutical solutions.
Preparing a Buffer
- Determine the optimal pH for the product, based on physical and chemical stability, therapeutic activity and patient comfort and safety (must consider chemical and physical nature of the active and other ingredients and the route administration).
- Select a weak acid with a pKa near the desired pH (must be nontoxic and physically/chemically compatible with other solution additives).
- Calculate the ratio of salt to acid required to produce the desired pH (Henderson-Hasselbach equation).
- Determine the desired buffer capacity of the product (consider stability of product, route of administration, volume of dose, chemical nature of product).
- Calculate the total buffer concentration required to produce this buffer capacity (Van Slyke equation).
- Determine the pH and the buffer capacity of the completed buffer solution by using a reliable pH meter or pH paper. (This may not always be practical, especially when small volume, sterile products are prepared.)
sample calculation:
Using Acetic Acid and Sodium Acetate prepare 500 ml of a buffer solution at pH 4.5 with a buffer capacity of 0.05.

Salt to Acid Ratio:

![]()

![[salt] = 0.55 [acid]](https://pharmlabs.unc.edu/labs/ophthalmics/images/ophthalmics-prepare-eq5.gif)
Total Buffer Concentration:

![[H3O+] = antilog(- pH) = antilog(- 4.5)](https://pharmlabs.unc.edu/labs/ophthalmics/images/ophthalmics-prepare-eq7.gif)


![]()
Final Calculations:
![]()
![C = 0.55 [acid] + [acid] = 1.55 [acid] = 0.095 M](https://pharmlabs.unc.edu/labs/ophthalmics/images/ophthalmics-prepare-eq12.gif)
![[acid] = 0.095 M ÷ 1.55 = 0.061 M or 0.061 moles/L × 0.5 L × 60 g/mole = 1.85 g acetic acid](https://pharmlabs.unc.edu/labs/ophthalmics/images/ophthalmics-prepare-eq13.gif)

![[salt] = 0.55 [acid] = 0.55 × 0.061 M = 0.034 M or 0.034 moles/L × 0.5 L × 82 g/mole = 1.38 g sodium acetate](https://pharmlabs.unc.edu/labs/ophthalmics/images/ophthalmics-prepare-eq15.gif)
Iso-osmoticity and Isotonicity
If a semi-permeable membrane (one that is permeable only to solvent molecules) is used to separate solutions of different solute concentrations, a phenomenon known as osmosis occurs in which solvent molecules cross the membrane from lower to higher concentration to establish a concentration equilibrium. The pressure driving this movement is called osmotic pressure and is governed by the number of “particles” of solute in solution. If the solute is a nonelectrolyte, the number of particles is determined solely by the solute concentration. If the solute is an electrolyte, the number of particles will be governed by both the concentration and degree of dissociation of the substance.
Solutions containing the same concentration of particles and thus exerting equal osmotic pressures are called iso-osmotic. A 0.9% solution of NaCl (Normal Saline) is iso-osmotic with blood and tears. The term isotonic, meaning equal tone, is sometimes used interchangeably with the term iso-osmotic. The distinction between these terms comes with the realization that red blood cell membranes are not perfect semipermeable membranes, but allow passage of some solutes, such as alcohol, boric acid, ammonium chloride, glycerin, ascorbic acid, and lactic acid. Hence, a 2% solution of boric acid while physically measured to be iso-osmotic (containing same number of particles) with blood, will not be isotonic (exerting equal pressure or tone) with blood but is isotonic with tears. Practically speaking, this differentiation is rarely of significance and isotonicity values calculated on the basis of the number of particles in solution is usually sufficient.
The clinical significance of all this is to insure that isotonic or iso-osmotic solutions do not damage tissue or produce pain when administered. Solutions which contain fewer particles and exert a lower osmotic pressure than 0.9% saline are called hypotonic and those exerting higher osmotic pressures are referred to as hypertonic. Administration of a hypotonic solution produces painful swelling of tissues as water passes from the administration site into the tissues or blood cells. Hypertonic solutions produce shrinking of tissues as water is pulled from the biological cells in an attempt to dilute the hypertonic solution. The effects of administering a hypotonic solution are generally more severe than with hypertonic solutions, since ruptured cells can never be repaired. The eye can tolerate a range of tonicities as low as 0.6% and as high as 1.8% sodium chloride solution.
Several methods are used to adjust isotonicity of pharmaceutical solutions. One of the most widely used method is the sodium chloride equivalent method. The NaCl equivalent (E) is the amount of NaCl which has the same osmotic effect (based on number of particles) as 1 gm of the drug.
sample calculation: Calculate the amount of NaCl required to make the following ophthalmic solution isotonic.
| Rx
Atropine Sulfate 2% |
1. Determine the amount of NaCl to make 30 ml of an isotonic solution
2. Calculate the contribution of atropine sulfate to the NaCl equivalent
3. Determine the amount of NaCl to add to make the solution isotonic by subtracting (2) from (1)
Other substances may be used, in addition to or in place of NaCl, to render solutions isotonic. This is done by taking the process one step further and calculating the amount of the substance that is equivalent to the amount of NaCl calculated in step 3.
For example, boric acid is often used to adjust isotonicity in ophthalmic solutions because of its buffering and anti-infective properties. If E for boric acid is 0.50, then the amount of boric acid needed to replace the NaCl in step 3 can be calculated:
![]()
or ![]()
or, more simply: ![]()
Thus, 0.38 g or 380 mg of boric acid would be required to render the previous ophthalmic solution isotonic.
Isotonic Buffers
The addition of any compound to a solution will affect the isotonicity since isotonicity is a property of the number of particles in solution. So the osmotic pressure of a solution will be affected not only by the drug but also by any buffer compounds that are included in the formulation. But after these compounds have been added, it is still possible that the solution will not be isotonic. It may be necessary to add additional sodium chloride to bring the solution to isotonicity, but that would require doing the calculations as shown above.
An alternative to this approach is to use an isotonic buffer. There are two approaches to using isotonic buffers.
Approach 1
In the first approach, the drug is dissolved in an appropriate volume of water (V-value) to make the solution isotonic. Then the remaining volume needed in the formulation is supplied by an isotonic buffer
An example:
| Rx Procaine HCl 2% Aqua. dest. q.s. ad 15 ml M.Ft. Isotonic, buffered injection |
The formulation requires 0.3 g of Procaine HCl. V-value tables can been found in standard references and are tabulated to tell how many ml of water, when added to 0.3 g of drug, will result in an isotonic solution. For Procaine HCl, 7 ml of water added to 0.3 g of drug will make an isotonic solution. Therefore 0.3 g Procaine HCl is dissolved in 7 ml water, and then sufficient buffered, isotonic vehicle of appropriate pH is added to make 15 ml.
The pH of an isotonic Procaine HCl solution is 5.6. Therefore, an isotonic buffer of approximately that pH would be used. One commonly used isotonic buffer is the Sorenson’s Modified Phosphate Buffer.
| ml of 0.0667 M NaH2PO4 |
ml of 0.0667 M Na2HPO4 |
Resulting pH | NaCl required for isotonicity (g/100ml) |
|---|---|---|---|
| 90 | 10 | 5.9 | 0.52 |
| 80 | 20 | 6.2 | 0.51 |
| 70 | 30 | 6.5 | 9.5. |
| 60 | 40 | 6.6 | 0.49 |
| 50 | 50 | 6.8 | 0.48 |
| 40 | 60 | 7.0 | 0.46 |
| 30 | 70 | 7.2 | 0.45 |
| 20 | 80 | 7.4 | 0.44 |
| 10 | 90 | 7.7 | 0.43 |
| 5 | 95 | 8.0 | 0.42 |
(from USP XXI, p. 1338)The closest Sorenson’s buffer to pH 5.6 would be pH 5.9. So to complete the formulation, 8 ml of pH 5.9 Sorensen’s buffer would be added to the 7 ml of Procaine HCl solution. The individual amounts of the Sorenson’s buffer to add can be determined:
Therefore, the compounding procedure would be to weigh 0.3 g Procaine HCl and 0.04 g NaCl, add 7 ml of H2O, 7.2 ml of 0.0667 M NaH2PO4, and 0.8 ml of 0.0667 M Na2HPO4. Filtration sterilize the solution and package in a sterile final container.
The limitation to this approach is that the final formulation pH may be different than the desired pH. The final pH will depend on the two pHs and buffer capacities of the Sorensen’s buffer and the aqueous drug solution.
Approach 2
The second method is to use the Sorensen’s buffer as the entire solvent of the formulation. In this situation, the sodium chloride equivalent of the active drug is subtracted from the “NaCl required for isotonicity” listed in the table. This method has the advantage that the pH of the final solution will be the pH of the selected Sorensen’s buffer.
An example:
| Ampicillin Sodium 30 mg/ml Sodium Chloride q.s. Make 15 ml of sterile, buffered, isotonic solution at pH 6.6 |
1. A ratio calculation will show that 0.45 g of Ampicillin Sodium is needed for this formulation.
2. The sodium chloride equivalent for Ampicillin Sodium is 0.16. Therefore, the drug will contribute osmotic pressure as if it was 0.072 g of sodium chloride.
3. To have a pH of 6.6, 9.0 ml of monobasic sodium phosphate solution and 6.0 ml of dibasic sodium phosphate solution are needed. Then to adjust the isotonicity, 0.0735 g of sodium chloride is needed, but the Ampicillin Sodium equivalent will account for 0.072 g. So an additional 0.0015 g of sodium chloride must be added.
Therefore, the compounding procedure would be to weigh 0.45 g of Ampicillin Sodium and 0.0015 g of sodium chloride. Add 9.0 ml of monobasic sodium phosphate solution and 6.0 ml of dibasic sodium phosphate solution. Filtration sterilize the solution and package in a sterile final container.
Preservatives
Ophthalmic solutions are generally packaged in multiple dose containers. Since there is the possibility of inadvertent bacterial contamination of the formulation with repeated patient use, a preservative should be added. Preservatives should be used that do not cause patient sensitivity or that are incompatible with the other ingredients in the formulation.
Preservatives that are commonly used in ophthalmic formulations are listed in the table below. The FDA Advisory Review Panel on OTC Ophthalmic Drug Products (Dec. 1979) established that the concentrations are for formulations that will have direct contact with the eye and not for ocular devices such as contact lens products.
| Agent | Maximum Concentration |
|---|---|
| Benzalkonium chloride
Benzethonium chloride Chlorobutanol Phenylmercuric acetate Phenylmercuric nitrate Thimerosal Methylparaben Propylparabens |
0.013%
0.01% 0.5% 0.004% 0.004% 0.01% 0.1- 0.2% 0.04% |
FDA Advisory Review Panel on OTC Ophthalmic Drug Products, Final report, Dec. 1979.
Preservatives do not immediately produce sterility and should not be the sole means of sterilizing a product. Patients should be counseled that the product may be easily contaminated by touching it to the eyes. Self-contained dropper bottles are less likely to be contaminated than those which must be opened and the dropper removed. However, the plastics used to make these are reactive with a number of solutions and may not be as acceptable as glass bottles.
Antioxidants
Some drugs can be chemically degraded by oxidation. If such a drug is present in the formulation, an antioxidant should be added. The common agents and their maximum concentration used in ophthalmic formulations are in the table below. Sulfites can cause allergic-type reactions in certain people and so patients should be questioned about this potential reaction before the antioxidant is included in the formulation. Ethylenediaminetetraacetic acid (also known as Edetic Acid or EDTA) is not often used in ophthalmic formulations because of its low water solubility. However, disodium Edetate, the disodium salt of EDTA, is used instead because of its high water solubility.
| Antioxidant | Maximum Concentration (%) |
|---|---|
| Ethylenediaminetetraacetic acid | 0.1 |
| Sodium bisulfite | 0.1 |
| Sodium metabisulfite | 0.1 |
| Thiourea | 0.1 |
Viscosity
Viscosity measures the resistance of a solution to flow when a stress is applied. The viscosity of a solution is given in poise units. The unit centipoise (cp or the plural cps) is equal to 0.01 poise and is most often used in pharmaceutical applications. Compounds used to enhance viscosity are available in various grades such as 15 cps, 100 cps, etc. The grade number refers to the viscosity that results when a fixed percentage aqueous solution is made. Generally the solutions are 1% or 2% and the viscosity is measured at 20oC.
Viscosity enhancers are used in ophthalmic solutions to increase their viscosity. This enables the formulation to remain in the eye longer and gives more time for the drug to exert its therapeutic activity or undergo absorption. Commonly used viscosity enhancers and their maximum concentrations are given in the table below.
| Viscosity Enhancer | Maximum Concentration (%) |
|---|---|
| Hydroxyethylcellulose | 0.8 |
| Hydroxypropylmethylcellulose | 1.0 |
| Methylcellulose | 2.0 |
| Polyvinyl alcohol | 1.4 |
| Polyvinylpyrrolidone | 1.7 |
The most common viscosity desired in an ophthalmic solution is between 25 and 50 cps. The actual concentration of the enhancer required to produce that viscosity will depend on the grade of the enhancer. For example, if methylcelluse 25 cps is used, a 1% solution will create a viscosity of 25 cps. If methylcellulose 4000 cps is used, a 0.25% solution provides the desired viscosity. Standard references give tables of viscosities produced by percentage solutions and grades of ingredients.
Sterility
Sterility is defined as the absence of viable microbial contamination. Sterility is an absolute requirement of all ophthalmic formulations. Contaminated ophthalmic formulations may result in eye infections that could ultimately cause blindness, especially if the Pseudomonas aeruginosa microbe is involved. Therefore, ophthalmic formulations must be prepared in a laminar flow hood using aseptic techniques just the same as intravenous formulations. The sterile formulations must be packaged in sterile containers.
View video demonstrations on how to how to use filters on syringes and using a membrane filter
Suspensions should not be filtered as the drug will be removed by the filter. Obviously, ointments cannot be filtered. For these formulations, the individual ingredients are sterilized separately and then they are formulated using aseptic techniques.
Artificial Tears: scenario and formulation record.