Radiopharmaceuticals: Labs
Laboratory Procedure
A. Preparation of Tc-99m Sulfur Colloid Injection
View a video demonstration on making a technetium-99m sulfur colloid
- Put on disposable gloves.
- Cover the work bench with plastic backed adsorbent paper.
- Prepare a boiling water bath (200 ml water in a 400 ml beaker) containing a lead shield. (The water level must be sufficient to cover the lead shield).
View a video demonstration on a rolling, boiling water bath
- In a 3 ml syringe, aseptically obtain 1 ml of sodium pertechnetate Tc-99m solution and sign the log out sheet. Assay the dose in the syringe with the radionuclide dose calibrator. Record the activity and time the dose was measured, as well as the activity and time of calibration of the original sample from the generator. Calculate the activity that should be present using the decay table provided and compare the calculated activity with the assayed activity.
- Swab the rubber closure of the sulfur colloid reaction vial. Place the reaction vial containing the lyophilized sodium thiosulfate, disodium EDTA, and gelatin into a lead pig.
- Inject the 1 ml of sodium pertechnetate Tc-99m solution into the reaction vial. DO NOT PRESSURIZE THE VIAL. Swirl vial to dissolve powder.
- Swab the rubber closure of vial A. Remove 1.5 ml of 0.148 N hydrochloric acid from vial A and aseptically inject the entire contents into the reaction vial. DO NOT PRESSURIZE THE VIAL. Swirl reaction vial to mix.
- Transfer the reaction vial to the lead shielded boiling water bath and heat for 5 minutes. The vial will float in the water bath.
- At the end of heating, remove the reaction vial and place it into a lead pig to cool for 3 minutes. Swab the reaction vial closure again.
- Swab the rubber closure of vial B. Remove 1.5 ml of phosphate buffer from vial B and aseptically transfer its contents into the reaction vial. DO NOT PRESSURIZE THE VIAL. Swirl reaction vial to mix.
- Calculate the radioconcentration (C) of the final 99mTc-SC using the following formula:
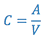
where:- A = Tc-99m activity added to the reaction vial at the time indicated on the label (Assayed Activity).
- V = Total volume in the reaction vial
- Complete the information on the pressure-sensitive label and place this label on the lead pig.
B. Radiochromatography (for radiochemical purity)
View a video demonstration on determining the radiopurity of a sulfur colloid
- Obtain three, Whatman 3-MM paper strips. Make a very light pencil mark 1 cm from one end of each strip to mark the origin. Place an ink mark with a water-based flair pen 2 mm from the opposite end to mark the solvent front. Number the top and bottom of each strip with the pencil as follows:

- Using a clean 1 ml syringe and 27 gauge needle obtain a 0.1 ml sample of your 99mTc-SC preparation. Place a one drop sample (2 – 3 mm diameter spot) on the pencil line at the origin on strip 1.
- Repeat Step 2 using 99mTc pertechnetate dilution on strip 2 and the unknown 99mTc-SC on strip 3.
- Place each strip, origin end down, into a scintillation vial containing 0.9% Sodium Chloride Solution which is 2 – 3 mm deep. Be sure spot is above the level of liquid as follows:

- Develop each strip until the solvent reaches the top, indicated by spreading of the ink spot.
- Remove the developed strips with forceps and blot dry on a paper towel. Cut each strip into two equal pieces.
- Obtain 30 second background count before counting your samples. Place each strip half onto a counter tray and count for 30 seconds with a gamma scintillation counter.
- Determine the net count per minute (CPM) per strip by subtracting the background CPM from each sample CPM. Record the data in your handout.
Comments: Radiochemical purity of a radiopharmaceutical is defined as the fraction of the total radioactivity present in the desired chemical form. Strip number 2 demonstrates the migration characteristics of the impurity, Tc-99m pertechnetate. It migrates to the solvent front, whereas 99mTc-SC remains at the origin.
_________________________________________________________________________________________________________________________
Experimental Data and Results
A. Preparation of Tc-99m Sulfur Colloid Injection
| Calibration time for sample from generator | __________________ |
| Calibrated activity at this time for sample from generator | __________________ µCi |
| Time your sample’s activity was assayed in lab | __________________ |
| Assayed activity (from 1 ml sample) | __________________ µCi |
| Calculated activity in 1.0 ml of sodium pertechnetate Tc-99m solution | __________________ µCi |
| Total volume of the final 99mTc-SC product | __________________ ml |
| The radioconcentration (C) of the final 99mTc-SC product (from assayed activity) | __________________ µCi/ml |
DISPOSE OF ALL MATERIALS IN SPECIALLY MARKED SAFETY CONTAINERS. DO NOT PLACE IN TRASH.
B. Radiochromatography
Background CPM _________________
| Sample | Counts/30 sec | Total CPM | Net CPM | % of Total Activity |
|---|---|---|---|---|
| 1. Product O |
||||
| SF | ||||
| O + SF | XXX | XXX | 100% | |
| 2. Pertechnetate ion O |
||||
| SF | ||||
| O + SF | XXX | XXX | 100% | |
| 3. Unknown product O |
||||
| SF | ||||
| O + SF | XXX | XXX | 100% |
O = Origin, SF = Solvent Front
What is the radiochemical purity of your product?___________________
What is the radiochemical purity of the unknown? ___________________
Inspection of Final Product/Label ___________________________________
Discussion Questions
- Would you dispense your 99mTc-SC product for patient use? The 99mTc-SC unknown product? Explain.
- You have just received the following order in the pharmacy. What volume of your 99mTc-SC preparation is required to fill the order if the dose is prepared 3 hours after the product calibration time? Half life for Tc-99m is 6 hours. Show your calculations!
For: John Jones
Rx: Tc-99 Sulfur Colloid 5 µCi for liver scan
Sig: Dispense one dose