Extraction of Drugs
Upon completion of this exercise, you should be able to:
- Describe three (3) pharmaceutical applications of the principles of extraction and partition coefficient.
- Define solvent extraction and describe how it is most efficiently carried out.
- Given the solvent extraction equations, the partition coefficient for the system, and the relative volumes of original and extracting solvents, calculate the weight or fraction of drug extracted after an number of extractions or calculate the number of extractions required to extract a specified fraction of the drug.
- To measure the extraction of salicylic acid from aqueous buffer solution into two different organic solvent mixtures.
- To measure the extraction of salicylic acid (0.02%) from aqueous buffer of various pH’s and thereby determine the pKa for salicylic acid.
If a compound is added to a mixture of two immiscible liquids, it will become distributed between the two layers in a definite concentration ratio. If C1 and C2 are the equilibrium concentrations of the compound in solvents 1 and 2 respectively,
then

where K is the equilibrium constant or partition coefficient.
This equation applies only when the molecules in each phase are in the same state of aggregation. When association and dissociation occurs, more complex equations must be applied.
No convention exists regarding whether the concentration in the organic phase or aqueous phase should be placed in the numerator to determine K. One should always specify which way the partition coefficient is being expressed. For example:

The concept of partition coefficient is applied in the pharmaceutical and health care industries in a variety of ways, including solvent extractions of drugs from dosage forms (e.g. extraction of aspirin from tablets for quality assurance tests), drugs from biological fluids (e.g. clinical laboratory analyses of drugs in blood/plasma or urine), and medicinal agents from plants (e.g. the extraction of vinca alkaloids in the development of cancer chemotherapy agents). Solvent extraction is the process of removing a constituent from one liquid phase by bringing this phase into contact with a second, immiscible, liquid phase. The number of extractions required to isolate or purify a substance is determined by the partition coefficient (K) and the relative volumes of the two phases.
At equilibrium, the weight of solute remaining in the original solvent after a single extraction (W) or after multiple extractions (Wn) is:

where,

e.g. A 25 ml sample of an aqueous solution containing 0.1 g of acetanilid was extracted with three 10 ml portions of ether. The combined ether extracts were evaporated to dryness and the residue weighed. The ether/water partition coefficient for acetanilid is 3.0. What is the weight of the residue (i.e. how much is extracted)?
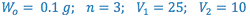
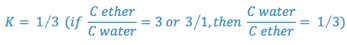
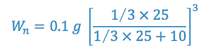

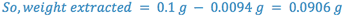
The most efficient extraction results when a large number of extractions are carried out with small portions of extracting liquid.
Most of the compounds encountered in pharmaceutical analysis are weak acids or bases and their solubility characteristics depend upon their ionic form (molecular species being more soluble in nonpolar, organic solvents and the ionic species being more soluble in polar especially aqueous solvents). Neutral or unionized compounds can be extracted from aqueous solutions while ions cannot. When a drug exists in its ionized form, it can be converted to the unionized form by altering the pH of the medium in which the drug is contained.
1. Partition coefficient as a function of pH
Many drugs are acids or bases that dissociate in aqueous solution. Changing the pH of the aqueous solution alters the ratio between the ionized and unionized form of the drugs. Since it is the unionized form which is extracted into the organic phase, the fraction extracted will vary with pH of the aqueous solution.
In aqueous buffer:

The partition between organic and aqueous buffer can be described by a “true” partition coefficient, PC, as:

In lab you will be measuring an apparent partition coefficient, PC’, which will vary with pH or [H3O+]. This apparent partition coefficient is given by

2. The partition coefficient and pKa determination
It is possible to determine the pKa of the drug undergoing extraction using the data generated in part B.1.
Using the equation:

and substituting PC.[HA]org for [HA]org and Ka[HA]aq/[H3O+] for [A–]aq, gives the following equation:

which simplifies to

This gives us an equation with PC’ as function of [H3O+] with two unknown parameters, PC and Ka. This can be converted into a straight line equation by taking the reciprocal of both sides of the equation.
Thus:

or
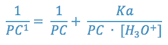
Therefore plotting 1/PC’ versus 1/[H3O+] should give a straight line with a slope of Ka/PC and an intercept of 1/PC.