Tablets: Labs
Learning Objectives
A. Determination of Tablet Hardness
Using a hardness tester, determine the hardness of the four (4) different types of tablets supplied. Use an average of three measurements for each determination and comment on your results.
View a video demonstration on testing tablet hardness
Hardness Tester _______________
| Tablet Type | Hardness 1 | Hardness 2 | Hardness 3 | Mean Hardness |
|---|---|---|---|---|
Comment on Results
B. Determination of Uniformity of Dosage Units
- Determination of Weight Variation
The total weight of a tablet is determined by the depth of the die cavity, bulk density of granules or powder, and uniformity of particulate flow. Even with a proper granulation having uniform flow, a volume fill is not as accurate as a fill based on weight. Therefore, tablet weight variations must fall within certain specifications established by the USP.Using your prescription balance, determine the individual weights of ten of the thirty calcium carbonate tablets provided. Take care to preserve the identity of each tablet.
| Tablet # | Weight of Tablet (mg) | % Labeled Claim |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 7 | ||
| 8 | ||
| 9 | ||
| 10 |
Note: Labeled claim is 750 mg
Mean (as % of labeled claim) __________
Standard Deviation __________
RSD __________
b. Determination of Content Uniformity
NOTE: Tablets should be analyzed in the same order as the weight variation test so that a comparison between weight variation and content uniformity can be made.
Determine the content of active ingredient of the tablets by the procedure given below. The tablets are labeled to contain 648 mg of calcium carbonate.
Procedure: In an Erlenmeyer flask, pipet 25 ml of approximately 1 N HCl (note exact normality) and carefully drop a tablet into the solution. Lightly agitate the solution and gently warm it to ensure complete dissolution and to drive off the generated carbon dioxide. The excess HCl is back-titrated using NaOH solution (note exact normality). Use four drops of phenolphthalein TS as indicator.
Calculate the amount of calcium carbonate in each tablet.
SAMPLE CALCULATION
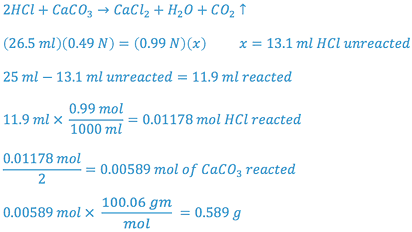
| Tablet # | ml___ N NaOH Used | ml___ N HCl Unreacted | ml___ N HCl Reacted with CaCO3 | Amount CaCO3 Per Tablet (mg) | % Labeled Claim |
|---|---|---|---|---|---|
| 1 | |||||
| 2 | |||||
| 3 | |||||
| 4 | |||||
| 5 | |||||
| 6 | |||||
| 7 | |||||
| 8 | |||||
| 9 | |||||
| 10 |
Note: Labeled claim is 648 mg
Mean Weight of Active Ingredient _____________
Mean (as % of labeled claim) ______________
Standard Deviation ___________________
RSD _______________________________
C. Discussion
- Discuss the hardness of the four (4) types of tablets you tested (i.e. are they appropriate for the intended use of the tablet?)
- Refer to USP monograph for Calcium Carbonate.
- What margin of error (content) is allowed in the USP specifications?
- Do the tablets you assayed pass the criteria for the first part (first 10 units) of the weight variation test?
- Do they pass the content uniformity test?
- Is there a correlation between weight variation and content uniformity in the tablets you analyzed? How did you determine if a correlation existed? How would you account for variations from the expected correlation?