Suspensions: Labs
Laboratory Procedure
Complete parts A, B, and C in groups of 2; complete part D individually.
A. Effect of Electrolyte Concentration on Sedimentation Parameters
- Prepare 100 ml each of a series of suspensions containing 4% bismuth subnitrate and 6 varying concentrations of monobasic potassium phosphate in 100 ml graduated cylinders. A 10% KH2PO4 stock solution will be provided. Use glycerin (approximately 15 drops) as a levigating agent.
View a video demonstration on making a suspension for the experiment
View a video demonstration on how to levigate a powder
| Suspension # | Conc. of KH2PO4/ Bismuth Subnitrate |
Volume of 10% KH2PO4Stock Solution (ml) | Weight of Bismuth Subnitrate (g) | Volume of Water (ml) |
|---|---|---|---|---|
| 1 | 0.00% / 4% | |||
| 2 | 0.01% / 4% | |||
| 3 | 0.05% / 4% | |||
| 4 | 0.1% / 4% | |||
| 5 | 0.5% / 4% | |||
| 6 | 1.0% / 4% | |||
| 7 | 5.0% / 4% |
- Determine the sedimentation rate of each suspension. Shake the suspension vigorously making sure all of the particles are uniformly suspended, and note the time. Observe the boundary between the sediment and the supernatant and record the time it takes for the boundary to pass each 10 ml graduation until the volume of sediment has reached 30 ml. The best way to observe the boundary is to view it directly in front of a light source. You might try viewing it with sunlight from the windows as your light source. You should note whether there is a clear and distinct boundary or no obvious boundary. Record your data in Table I.
- Plot the volume of sediment vs. time and draw the best straight line. The slope will be equal to the sedimentation rate.
- Redisperse and allow each suspension to sit undisturbed for 24 hours. Then, determine and record the final volume of sediment.
- Estimate the degree of caking in each system. After allowing the suspensions to sit for 3 or 4 days, determine the number of times the bottle must be inverted to resuspend all of the particles.
View a video on the overview of experimental results
| Concentration of KH2PO4 |
Distinct Boundary (Y/N) |
||
|---|---|---|---|
| 0.00% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr | |
| 0.01% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr | |
| 0.05% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr | |
| 0.10% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr | |
| 0.50% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr | |
| 1.0% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr | |
| 5.0% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr |
|
Note: If no sedimentation is apparent after several minutes, record sedimentation rate as “zero” or “too slow to measure” and go on to next product. |
B. Effect of Thickening Agents on Sedimentation Rate
- Prepare 350 ml of a 0.4% stock solution of CMC (carboxymethylcellulose). In a beaker calibrated to 350 ml, heat 300 ml of water until boiling. Add required amount of CMC slowly with constant stirring. Continue to heat until a clear homogeneous solution is obtained. Cool and q.s. to 350 ml with distilled water.
- Prepare 100 ml each of a series of suspensions containing 4% bismuth subnitrate, 0.2% CMC and varying concentrations of monobasic potassium phosphate (see Table II). Use glycerin (approximately 15 drops) as a levigating agent.
- Plot the volume of sediment as a function of time and determine the sedimentation rate for each suspension. Sedimentation rate will be equal to the slope of the line.
- Redisperse and allow each suspension to sit undisturbed for 24 hours. Then, determine and record the final volume of sediment.
- After storing all the suspensions for a period of 3 to 4 days, determine the ease of redispersion in each system and determine which system is most acceptable.
| Concentration of KH2PO4 |
Distinct Boundary (Y/N) |
||
|---|---|---|---|
| 0.00% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr | |
| 0.01% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr | |
| 0.1% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr | |
| 1.0% | Volume of sediment (ml) 90 80 70 60 50 40 30Time (sec) |
24 hr |
|
Note: If no sedimentation is apparent after several minutes, record sedimentation rate as “zero” or “too slow to measure” and go on to next product. |
C. Summary
| % KH2PO4 | % CMC | Sedimentation Rate (ml/sec)1 | Final vol. (ml)2 | Sedimentation vol.3 | Degree of Flocculation4 | Ease of Redispersion (# of inversions)5 |
|---|---|---|---|---|---|---|
| Part A Table I 0.00 |
0 | |||||
| 0.01 | 0 | |||||
| 0.05 | 0 | |||||
| 0.10 | 0 | |||||
| 0.50 | 0 | |||||
| 1.0 | 0 | |||||
| 5.0 | 0 | |||||
| Part B Table II 0.00 |
0.2 | |||||
| 0.01 | 0.2 | |||||
| 0.1 | 0.2 | |||||
| 1.0 | 0.2 |
Footnotes:
a. Sedimentation Rate – calculated from slopes of graphs
b. Final volume – From Table I and II (sedimentation volume at 24 hours)
c. Sedimentation volume:

d. Degree of flocculation:
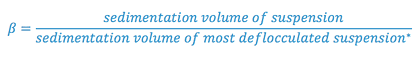
-
- * system with smallest sedimentation volume
e. Record the number of times the bottle must be inverted to resuspend all the particles (suspensions to sit for 3 to 4 days).
- Compare the sedimentation rates for suspensions prepared with and without CMC, but containing monobasic potassium phosphate.
- Compare the sedimentation rates for suspensions prepared with and without CMC, but containing no monobasic potassium phosphate.
- Plot the sedimentation volume and sedimentation rate as a function of the logarithmic value of the electrolyte concentration using your data from Part A. Plot concentration on the x-axis in logarithmic scale, sedimentation volume on the left linear y-axis, and sedimentation rate on the right linear y-axis.
- The range of electrolyte concentration in which a controlled flocculation occurs is ______________________ (interpretation of graph).
D. Preparation of Pharmaceutical Suspensions
Compound and dispense the following prescriptions according to the formulations given in Remington’s.
Owen Mealot, MD Rx # 61201
123 Upendown Rd.
Nowhere, NC 27000
Phone: 555-1234 DEA # AH0079411
Name:Ima Iching Date: Today
Address: 123 Della Street Phone: 555-5678
Rx
Calamine Lotion 60 ml
Sig: Apply to affected areas 3-4 times a day
Refills: 1
Owen Mealot, MD
Product selection permitted Dispense as written
|
Owen Mealot, MD Rx # 61202
123 Upendown Rd.
Nowhere, NC 27000
Phone: 555-1234 DEA # AH0079411
Name:Ima Iching Date: Today
Address: 123 Della Street Phone: 555-5678
Rx
White Lotion 60 ml
Sig: Apply to affected areas 3-4 times a day
Refills: 1
Owen Mealot, MD
Product selection permitted Dispense as written
|
Preparation Guidelines for Calamine Lotion
Show the formula for 60 ml of Calamine Lotion.
Outline the compounding procedures for the suspension.
Inspection of Final Product_____________
Preparation Guidelines for White Lotion
Show the formula for 60 ml of White Lotion.
Outline the compounding procedures for the suspension.
Inspection of Final Product_____________
E. Discussion and Conclusions
- a. How does KH2PO4 induce controlled flocculation?
b. Why is the concentration of KH2PO4 critical? - How does CMC contribute to the stability of the product.
- After storing the suspensions for a period of 3-4 days, determine the ease of redispersion of each. Based on all your observations (Tables I-III), which product would you consider to be most acceptable? Explain.
- Identify the role of each of the formulation ingredients in the calamine prescription you prepared.
- What are the compounds suspended in White Lotion?
- Observe the sedimentation rate in White Lotion. What could you do to retard that rate?