Capsules
Upon completion of this exercise, you should be able to:
- Describe the advantages and limitations of capsules as dosage forms.
- Describe the official USP tests required for capsules.
- Prepare and dispense hard gelatin capsules.
- Evaluate capsules as a dosage form by performing and analyzing data from official USP tests.
 Capsules are gelatin shells filled with the ingredients that make up an individual dose. Dry powders, semi-solids, and liquids that do not dissolve gelatin may be encapsulated. Capsules account for about 20% of all prescriptions dispensed.
Capsules are gelatin shells filled with the ingredients that make up an individual dose. Dry powders, semi-solids, and liquids that do not dissolve gelatin may be encapsulated. Capsules account for about 20% of all prescriptions dispensed.
Capsules have several advantages as pharmaceutical dosage forms:
- They may be used to mask the unpleasant tastes, aromas, or appearance of a drug.
- They allow powders to be dispensed in an uncompressed form, thus allowing for quicker dissolution and absorption of the drug following oral dosing (as compared with tablets).
- They offer the pharmacist versatility to prepare any dose desired for a variety of administration routes (e.g. oral, inhalation, rectal, or to be diluted for vaginal, rectal, oral or topical use).
- They may be easier than tablets for some people to swallow.
- They can be made to alter the release rate of the drug.
Their disadvantages or limitations include the following:
- They are easily tampered with (although techniques exist for preventing this).
- They are subject to the effects of relative humidity and to microbial contamination.
- They may be difficult for some people to swallow.
- More expensive (commercially).
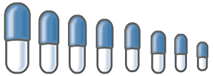
The hard gelatin capsule consists of a base or body and a shorter cap, which fits firmly over the base of the capsule. For human use, eight sizes of capsules are available. The capacity of each size varies according to the combination of drugs and their apparent densities. Capsules are available as clear gelatin capsules or in a variety of colors. The pharmacist can use the different colored capsules to distinguish two capsule formulations for the same patient, or to encapsulate unattractive ingredients. The pharmacist can add a dye to the powder before filling a clear capsule to impart a color for identification or esthetics.
Some types of hard gelatin capsules have a locking cap, which makes it more difficult to reopen the capsule.

To aid in the selection of the appropriate size, a table, with the capacity of five common drugs for that particular size capsule, is printed on the box of the capsules. As a guide, the relative sizes and fill capacities of capsules are given below. By knowing the bulk density of fill material, proper choice of capsule size is usually made easier; however, trial and error soon develops the judgment of the beginning pharmacist.
|
Capsule Size
|
Volume
(ml) |
Mg of Lactose
|
Mg of Aspirin
|
|---|---|---|---|
|
000
|
1.37
|
1340
|
1000
|
|
00
|
0.95
|
929
|
600
|
|
0
|
0.68
|
665
|
500
|
|
1
|
0.50
|
489
|
300
|
|
2
|
0.37
|
362
|
250
|
|
3
|
0.30
|
293
|
200
|
|
4
|
0.20
|
195
|
125
|
|
5
|
0.13
|
127
|
60
|
To hand fill capsules at the prescription counter, the pharmacist generally uses the “punch” method. The ingredients are triturated to the same particle size and then mixed by geometric dilution. The powder is placed on a powder paper or ointment slab and smoothed with a spatula to a height approximately half the length of the capsule body. The base of the capsule is held vertically and the open end is repeatedly pushed or “punched” into the powder until the capsule is filled; the cap is then replaced to close the capsule. Each filled capsule is weighed using an empty capsule as a counterweight. Powder is added or removed until the correct weight has been placed in the capsule. The filled capsule is tapped so that no air spaces are visible within the contents.
View a video demonstration on capsule punching
It is a good practice to remove from the stock container the exact number of empty capsules needed before you begin filling them. In this way you avoid preparing the wrong number of capsules and at the same time avoid contaminating the empty capsules with drug particles that cling to your hands. Also, since some fill material will likely be lost in the process of punching capsules, the pharmacist generally calculates for the preparation of at least one extra capsule to insure enough fill for the last capsule.
The simplest method by which a capsule may be kept free of moisture during compounding is to wash the hands well, dry them, and keep the fingers dry by stripping a towel through the cleansed fingers until warmth is felt. An alternative method is to use the base of one capsule as a holder for other bases during the filling operation. The capsules do not come in contact with the fingers. The most sure method of protecting the capsule is to wear finger cots or rubber gloves.
Capsule machines are available for filling 50, 100, and 300 capsules at a time. Each manufacturer’s machine is slightly different in its operation, but the series of operations is the same. Capsules are first loaded into the machine. Most machines come with a capsule loader which correctly aligns all of the capsules in the machine base. There are plates on the machine base that can be adjusted. First, the plates are adjusted to hold the capsule bodies in place while the caps are removed all at one time. The caps remain in place in the top of the machine for later use. Then the plates are adjusted again so that the capsule bodies will “drop” into place so that the tops are flush with the working surface of the plate.
The formulation powder is poured onto the plate and special spreaders and combs are used to fill the individual capsules. Some manufacturer’s have special shakers that will also help spread the powder and fill the capsules. The powder is spread evenly over the plate, and the comb is used to tamp and pack the powder into the capsules. These two processes are repeated over and over again until the capsule bodies are filled with the powder. All of the caps are then simultaneously returned to the capsule bodies, and the closed capsules are removed from the machine.
The machine has the advantage of filling many capsules in a timely manner. However, there is a tendency to pack the capsules in the middle of the plate with more powder than the capsules along the periphery. It takes practice to ensure that each capsule has the same amount of drug. A quality control procedure should be executed with each batch of capsules produced with the machine.
Using a Capsule Machine
 |
The parts of a capsule filling machine are: |
 |
To begin, the cap holder is placed on the base plates… |
 |
… and the capsule loader is filled with capsules. The loader will align the capsules so that the bottoms faces down in the base plate. The loader is used twice, since it only can hold 50 capsules at a time. |
 |
The cap holder contains the caps (shortest part) of the capsule, and the bodies of the capsules are in the base plates. |
 |
The plastic top of the cap holder is closed so the caps will not fall out when removed from the capsule bodies. The retention screws on the base plates are tighten to hold the capsule bodies as the cap holder is removed. |
 |
The cap holder is removed taking the caps with it … |
 |
…while the capsule bodies remain in the base plates. |
 |
The retention screws are loosen which allows the capsule bodies to completely descend into the base plates. |
 |
The powder mixture is poured onto the base plates, and the scraper is used to spread the powder over the capsules. |
 |
The comb is used to pack the capsules. The complete filling process is a secession of scraping and packing… |
 |
…until all are uniformly filled. It is quite common to complete a filling a capsule with a variation of less than 1%. |
 |
The cap holder is fitted back onto the base plates, and the capsule cap are pushed only the capsule bodies. |
 |
When the cap holder is removed, the capsules are removed from the base plates… |
 |
…and ready to be analyzed and dispensed. |
Once the capsules have been compounded and the capsule closed, the pharmacist may want to “seal” the capsule. The best way is to use “locking” capsules, where the body and cap lock together, making it very difficult to open the capsule again. If using locking capsules, during the filling process the cap is not completely closed onto the body in the weighing procedure to determine the weight of powder in the capsule. The locking is done only one time and that is after the capsule is correctly filled.
If locking capsules are not used, a seal can be made by touching the outer edge of the body with a moist towel to soften the gelatin. Alternatively, a cotton swab dipped in warm water can be rubbed around the inner edge of the cap. When the cap is closed on the body, it is slightly twisted to form the seal.
When compounding and sealing are complete, the capsules may need cleaning to remove fingerprints, traces of body oils, or loss powder from the capsule. Fingerprints and oils cannot be effectively cleaned from capsules so the best way to prevent these problems is to wear gloves during the compounding process. Any clinging powder can be removed by rolling the capsules between the folds of a towel.
Another proposed cleaning method is to put the capsules in a container filled with sodium bicarbonate, sugar, or sodium chloride, and gently roll the container. Then the container contents can be poured into a ten-mesh sieve where the “cleaning salt” will pass through the sieve.
Capsules should be visually inspected and checked for:
- Uniformity – check capsules for uniformity in appearance and color.
- extent of fill – check capsules for uniformity of extent of fill to ensure that all capsules have been filled.
- locked – check capsules to ensure that they have all been tightly closed and locked.
Section <795> of the USP 24/NF19 Supplement 1 requires that the capsule, “shall not be less than 90% and not more than 110% of the theoretically calculated weight of each unit.” This “weight variation” requirement (discussed in Section <905> of the USP 24/NF19) measures the variability in the amount of powder contained in each capsule. This procedure can be carried out in all pharmacies.
The other Dosage Form Uniformity test of Section <905> is “content uniformity” which measures the variability in the amount of active drug contained in each capsule. Most pharmacies are not equipped to carry out content uniformity analyses since special analytical equipment is required.
It is possible to have capsules that pass the weight variation requirement but not have content uniformity. This can occur if the material put into the capsules is not a homogenous mixture of all the ingredients. Some capsules would then have more active drug than other capsules. Appropriate mixing (i.e., geometric dilution) of all capsule ingredients into a homogenous mixture before filling the capsules. In this manner, the weight variation data will be sufficient to ensure the quality of the capsules.
Capsules are made of gelatin, sugar, and water and contain about 10% to 15% moisture. Gelatin can absorb up to ten times its weight in water. So if gelatin capsules are placed in areas of high humidity, they will become malformed or misshapen as they absorb moisture. On the other hand, if capsules are placed in low humidity, they become dry and brittle and may crack. To protect capsules from the extremes of humidity, they should be dispensed in plastic or glass vials and stored in a cool, drug place. It appears that a storage relative humidity of 30% to 45% is best. Cotton can be placed in the top of the vial to keep the capsules from rattling.
If powders that are being mixed before encapsulation are very light and fluffy and “difficult to manage,” add a few drops of alcohol, water, or mineral oil. As an alternative, mix these powders in a plastic bag. If the powders seem to have a “static charge,” use about 1% sodium lauryl sulfate.
Magnesium stearate (less than 1%) can be added to powders to increase their “flowability” which makes filling capsules easier. However, magnesium stearate is a hydrophobic compound and may interfere with the dissolution of the powders.
Hydroxypropylmethylcellulose, or Methocel, is a cellulose derivative polymer that is used as a hydrophilic matrix material. When Methocel hydrates, it forms a gel of such consistency that drug diffusion through the gel can be controlled. A hydrophilic matrix controlled release system is a dynamic system composed of polymer wetting, polymer hydration, and polymer dissolution. At the same time, other soluble excipients or drugs will also wet, dissolve, and diffuse out of the matrix while insoluble materials will be held in place until the surrounding polymer erodes or dissolves away.
Initially, the surface becomes wet and Methocel polymer starts to partially hydrate forming a gel layer on the surface of the capsule. As water continues to permeate into the capsule, the gel layer becomes thicker, and soluble drug will diffuse out through the gel layer. Ultimately, water will dissolve the capsule shell and continue to penetrate into the drug core. So release is controlled by the dissolution of soluble drug into the penetrating water and diffusion across the gel layer.
To formulate a successful hydrophilic matrix system, the polymer substance must wet and hydrate to form a gel layer fast enough to protect the interior of the capsule from dissolving and disintegrating during the initial wetting and hydration phase. If the polymer is too slow to hydrate, gastric fluids may penetrate to the capsule core, dissolve the drug substance, and allow the drug to diffuse out prematurely. Even among the family of hydroxypropylmethylcellulose products (Methocel E, F, and K), there are significant differences in the rate at which the polymers will hydrate. This is due to the varying proportions of the two chemical substituents attached to the cellulose backbone, hydroxypropoxyl and methoxyl substitution.
- The methoxyl substituent is a relatively hydrophobic component and does not contribute as greatly to the hydrophilic nature of the polymer and the rate at which it will hydrate.
- The hydroxpropoxyl group does contribute greatly to the rate of polymer hydration.
As a result, Methocel K products are the fastest to hydrate because they have the lower amount of the hydrophobic methoxyl substitution and a higher amount of the hydrophilic hydroxypropoxyl substitution. The range of chemical substitution in Methocel products is shown below.
| Product | % Methoxyl | % Hydroxypropoxyl | Relative Rate of Hydration |
|---|---|---|---|
| Methocel K | 19-24 | 7-12 | Fastest |
| Methocel E | 28-30 | 7-12 | Next Fastest |
| Methocel F | 27-30 | 4-7.5 | Slow |
| Methocel A | 27.5-31.5 | 0 | Slowest |
Increasing the concentration of the polymer in a matrix system increases the viscosity of the gel that forms on the capsule surface. Therefore, an increase in the concentration of the polymer used will generally yield a decrease in drug diffusion and drug release. An increase in the concentration of polymer also tends to put more polymer on the capsule surface. Wetting is more readily achieved so gel formation is accelerated.
This sample experiment was conducted in water and showed that after 2.5 hours, release was still continuing from the controlled release capsule.
| Conventional Capsule | Controlled Release Capsule | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clock Time | Run Time | Absorb. | Conc. | Cum. Amt. Released | Clock Time | Run Time | Absorb. | Conc. | Cum. Amt. Released | |
| 2:40 | 0 | 0.000 | 0.0 | 0.0 | 2:35 | 0 | 0.000 | 0.0 | 0.0 | |
| 2:55 | 15 | 0.005 | 0.0010 | 0.10 | 2:50 | 15 | 0.012 | 0.001 | 0.15 | |
| 3:10 | 30 | 0.016 | 0.0017 | 0.17 | 3:05 | 30 | 0.031 | 0.003 | 0.27 | |
| 3:25 | 45 | 0.031 | 0.0027 | 0.27 | 3:20 | 45 | 0.059 | 0.004 | 0.44 | |
| 3:40 | 60 | 0.048 | 0.0037 | 0.37 | 3:35 | 60 | 0.089 | 0.006 | 0.63 | |
| 3:55 | 75 | 0.065 | 0.0048 | 0.48 | 3:50 | 75 | 0.123 | 0.008 | 0.84 | |
| 4:10 | 90 | 0.592 | 0.037 | 3.75 | 4:05 | 90 | 0.160 | 0.011 | 1.07 | |
| 4:25 | 105 | 0.609 | 0.039 | 3.85 | 4:20 | 105 | 0.194 | 0.013 | 1.28 | |
| 4:40 | 120 | 0.610 | 0.039 | 3.86 | 4:35 | 120 | 0.240 | 0.016 | 1.56 | |
| 4:55 | 135 | 0.614 | 0.039 | 3.88 | 4:50 | 135 | 0.287 | 0.019 | 1.85 | |
| 5:10 | 150 | 0.612 | 0.039 | 3.87 | 5:05 | 150 | 0.302 | 0.019 | 1.95 | |
The same experiment was repeated in 0.01N HCl.
| Conventional Capsule | Controlled Release Capsule | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clock Time | Run Time | Absorb. | Conc. | Cum. Amt. Released | Clock Time | Run Time | Absorb. | Conc. | Cum. Amt. Released | |
| 2:20 | 0 | 0.000 | 0.0 | 0.0 | 2:15 | 0 | 0.000 | 0.0 | 0.0 | |
| 2:35 | 15 | 0.301 | 0.014 | 1.38 | 2:30 | 15 | 0.030 | 0.00044 | 0.044 | |
| 2:50 | 30 | 0.808 | 0.039 | 3.87 | 2:45 | 30 | 0.176 | 0.0076 | 0.76 | |
| 3:05 | 45 | 0.815 | 0.039 | 3.90 | 3:00 | 45 | 0.368 | 0.017 | 1.71 | |
| 3:20 | 60 | 0.822 | 0.039 | 3.94 | 3:15 | 60 | 0.516 | 0.024 | 2.48 | |
| 3:35 | 75 | 0.800 | 0.038 | 3.83 | 3:30 | 75 | 0.637 | 0.030 | 3.03 | |
| 3:50 | 90 | 0.800 | 0.038 | 3.83 | 3:45 | 90 | 0.740 | 0.035 | 3.54 | |
| 4:05 | 105 | 0.804 | 0.039 | 3.85 | 4:00 | 105 | 0.814 | 0.039 | 3.90 | |

The “rate of release” is determined by plotting the Cumulative Amount Released versus some function of time. For matrix diffusion controlled release, adaptations of the Higuchi equation are used; time is expressed as the square root of time and has units of minutes1/2. A linear trendline is fit through the points that occur after a lag time or before any asymptotic values are reached. The release of salicylic acid in 0.01N HCl is shown in the plot below. The conventional capsule apparently released all of its contents by 30 minutes, since after that time the amount released remained constant. The release rate was 1.55 mg/minutes1/2 which is almost three times as fast as seen with the controlled release capsule. But more importantly, the controlled release capsule continuously releases drug for hours, where the conventional capsule released all the drug within 30 minutes.
Tri-Estrogen Capsules: scenario and formulation record