Preparation of Suppositories
Upon completion of this exercise, you should be able to:
- List and describe a variety of suppository bases.
- Describe the proper formulation, packaging, and administration of suppository bases.
- Describe three (3) methods of suppository preparation.
- Prepare suppositories by the fusion (molding) technique.
- Determine the density factor of a drug in polyethylene glycol suppositories.
 Suppositories are medicated, solid bodies of various sizes and shapes suitable for introduction into body cavities. The medicament is incorporated into a base such as cocoa butter which melts at body temperature, or into one such as glycerinated gelatin or PEG which slowly dissolves in the mucous secretions. Suppositories are suited particularly for producing local action, but may also be used to produce a systemic effect or to exert a mechanical effect to facilitate emptying the lower bowel.
Suppositories are medicated, solid bodies of various sizes and shapes suitable for introduction into body cavities. The medicament is incorporated into a base such as cocoa butter which melts at body temperature, or into one such as glycerinated gelatin or PEG which slowly dissolves in the mucous secretions. Suppositories are suited particularly for producing local action, but may also be used to produce a systemic effect or to exert a mechanical effect to facilitate emptying the lower bowel.
The ideal suppository base should be nontoxic, nonirritating, inert, compatible with medicaments, and easily formed by compression or molding. It should also dissolve or disintegrate in the presence of mucous secretions or melt at body temperature to allow for the release of the medication. As with the ointment bases, suppository base composition plays an important role in both the rate and extent of release of medications.
Routes of Administration That Utilize Suppositories
Suppositories are medicated solid formulations that are inserted into body cavities. They are made in a variety of shapes and sizes because they are used in many different routes of administration (body cavities).
Rectal
Drugs administered via the rectum are given for a local effect or to achieve a systemic effect. Local effects may include the soothing of inflamed hemorrhoidal tissues, promoting laxation, and enemas. Using rectal administration to achieve systemic activity is preferred when the drug is destroyed in the GI tract, if oral administration is not possible because of vomiting, or the patient is unconscious or incapable of swallowing oral formulations. Rectal administration has been used to treat a variety conditions such as asthma, nausea, motion sickness, anxiety, and bacterial infections.
The most common rectal formulations are suppositories, solutions, and ointments. Suppositories are solid dosage forms that dissolve or melt when inserted into the rectum. Suppositories are manufactured in a variety of shapes. Rectal suppositories for adults are tapered at one end and usually weigh about 2 grams. Infant rectal suppositories usually weight about 1 gram or about half that of adult suppositories.
The major disadvantages of rectal suppositories:
- They are not preferred by patients; they are inconvenient.
- Rectal absorption of most drugs is frequently erratic and unpredictable.
- Some suppositories “leak” or are expelled after insertion.
Vaginal
Vaginal administration has many advantages.
- Generally there is less drug degradation via this route of administration compared to oral administration
- The dose can be retrieved if necessary
- There is the potential of long term drug absorption with various intrauterine devices (IUDs).
Vaginal administration does lead to variable absorption since the vagina is a physiologically and anatomically dynamic organ that causes pH and membrane permeability to change over time. There is also a tendency of some dosage forms to be expelled after insertion into the vagina.
Vaginal formulations include solutions, powders for solutions, ointments, creams, aerosol foams, suppositories, and tablets. Vaginal suppositories are employed as contraceptives, feminine hygiene antiseptics, bacterial antibiotics, or to restore the vaginal mucosa. Vaginal suppositories are inserted high in the vaginal tract with the aid of a special applicator. The suppositories are usually globular, oviform, or cone-shaped and weigh between 3 – 5 grams. Patients should be instructed to quickly dip the suppository in water before insertion. Because suppositories are generally used at bedtime and can be messy if the formulation is an oleaginous base, patients should wear a sanitary napkin to protect nightwear and bed linens.
Urethral
Urethral suppositories are not specifically described in the USP 24/NF19 either by weight or dimension. Traditionally, they are cylindrical in shape (3 – 6 mm in diameter) and vary in length according to gender. Female urethral suppositories can be 25 – 70 mm in length while male urethral suppositories can be about 50 – 125 mm in length. The one commercially available urethral suppository is actually marketed as a “pellet,” and is 1.4 mm in diameter and 3 or 6 mm in length depending on strength. Urethral suppositories are unusual and may not be encountered in a compounding practice.
Inserting Suppositories
Inserting Rectal Suppositories
- If possible, go to the toilet and empty bowels.
- Wash hands carefully with soap and warm water.
- Remove any foil or plastic wrapping from the suppository.
- Lubricate the tapered end of the suppository with a small amount of K-Y® Jelly. If the jelly is not available, moisten the suppository with a small amount of water.
- Either stand with one leg on a chair, or lay on one side with one leg straight and the other leg bent toward your stomach.
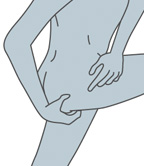 Standing Position |
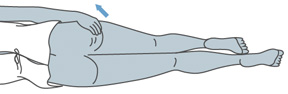 Laying Position |
- Separate buttocks to expose the rectal area.
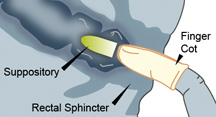
- Gently but firmly push the suppository into the rectum until it passes the sphincter (about 1/2 to 1 inch in infants, and 1 inch in adults.
- Close your legs and sit (or lay) still for about 15 minutes. Avoid emptying bowels for at least one hour (unless the suppository is a laxative). Avoid excessive movement or exercise for at least one hour.
- Wash hands again with soap and warm water immediately after inserting the suppository.
Inserting Vaginal Suppositories
- Wash your hands carefully with soap and warm water.
- Remove any foil or plastic wrapping from suppository.
- Place suppository in applicator.
- Hold the applicator by the opposite end from where the suppository is.
- Either lay on your back with your knees bent, or stand with your feet spread a few inches apart and your knees bent.
- Gently insert the applicator into the vagina as far as it will go comfortably. Once you are ready, push the inside of the applicator in and place the suppository as far back in the vagina as possible.
- Remove the applicator for the vagina.
- Wash your hands again with soap and warm water.
Suppository bases may be conveniently classified as according to their composition and physical properties:
|
 |
Oleaginous Bases
Oleaginous bases include Theobroma Oil and synthetic triglyceride mixtures.
 Theobroma Oil or cocoa butter is used as a suppository base because, in large measure, it fulfills the requirements of an ideal base. At ordinary room temperatures of 15° to 25°C (59° to 77°F), it is a hard, amorphous solid, but at 30° to 35°C (86° to 95°F), i.e., at body temperature, it melts to a bland, nonirritating oil. Thus in warm climates, theobroma oil suppositories should be refrigerated.
Theobroma Oil or cocoa butter is used as a suppository base because, in large measure, it fulfills the requirements of an ideal base. At ordinary room temperatures of 15° to 25°C (59° to 77°F), it is a hard, amorphous solid, but at 30° to 35°C (86° to 95°F), i.e., at body temperature, it melts to a bland, nonirritating oil. Thus in warm climates, theobroma oil suppositories should be refrigerated.
Particular attention must be given to two factors when preparing suppositories with cocoa butter base. First, this base must not be heated above 35°C (95°F) because cocoa butter is a polymorphic compound and if overheated will convert to a metastable structure that melts in the 25° to 30°C (77° to 86°F) range. Thus, the finished suppositories would melt at room temperature and not be usable.
The second factor is the change in melting point caused by adding certain drugs to cocoa butter suppositories. For example, chloral hydrate and phenol tend to lower the melting point. It may be necessary to add spermaceti or beeswax to raise the melting point of finished suppositories back to the desired range.
The newer synthetic triglycerides consist of hydrogenated vegetable oils. Their advantage over cocoa butter is that they do not exhibit polymorphism. They are, however, more expensive. Some of the bases are single entity formulations. Some of the names may denote a series of bases. In a series, the bases are varied to give a range of melting points. For example, Fattibase® is a single entity base that consists of triglycerides from palm, palm kernel, and coconut oils. Wecobee® is a series of bases. Wecobee FS, M, R, and S are all made from triglycerides of coconut oil. But FS has a melting point range of 39.4 to 40.5°C, M has a range of 33.3 to 36.0°C, R has a range of 33.9 to 35.0°C, and S has a range of 38.0 to 40.5°C. Other triglyceride type bases include Dehydag®, Hydrokote®, Suppocire®, and Witepsol®.
Water Soluble/Water Miscible Bases
Water soluble/water miscible bases are those containing glycerinated gelatin or the polyethylene glycol (PEG) polymers.
 Glycerinated Gelatin is a useful suppository base, particularly for vaginal suppositories. It is suitable for use with a wide range of medicaments including alkaloids, boric acid, and zinc oxide. Glycerinated gelatin suppositories are translucent, resilient, gelatinous solids that tend to dissolve or disperse slowly in mucous secretions to provide prolonged release of active ingredients.
Glycerinated Gelatin is a useful suppository base, particularly for vaginal suppositories. It is suitable for use with a wide range of medicaments including alkaloids, boric acid, and zinc oxide. Glycerinated gelatin suppositories are translucent, resilient, gelatinous solids that tend to dissolve or disperse slowly in mucous secretions to provide prolonged release of active ingredients.
Suppositories made with glycerinated gelatin must be kept in well-closed containers in a cool place since they will absorb and dissolve in atmospheric moisture. In addition, those intended for extended shelf-life should have a preservative added, such as methylparaben or propylparaben, or a suitable combination of the two. To facilitate administration, glycerinated gelatin suppositories should be dipped in water just before use.
 Polyethylene Glycol Polymers have received much attention as suppository bases in recent years because they possess many desirable properties. They are chemically stable, nonirritating, miscible with water and mucous secretions, and can be formulated, either by molding or compression, in a wide range of hardness and melting point. Like glycerinated gelatin, they do not melt at body temperature, but dissolve to provide a more prolonged release than theobroma oil.
Polyethylene Glycol Polymers have received much attention as suppository bases in recent years because they possess many desirable properties. They are chemically stable, nonirritating, miscible with water and mucous secretions, and can be formulated, either by molding or compression, in a wide range of hardness and melting point. Like glycerinated gelatin, they do not melt at body temperature, but dissolve to provide a more prolonged release than theobroma oil.
Certain polyethylene glycol polymers may be used singly as suppository bases but, more commonly, formulas call for compounds of two or more molecular weights mixed in various proportions as needed to yield a finished product of satisfactory hardness and dissolution time.
Since the water miscible suppositories dissolve in body fluids and need not be formulated to melt at body temperature, they can be formulated with much higher melting points and thus may be safely stored at room temperature.
Examples of various PEGs used in suppository bases are:
|
|
|
|
||||||||||||||||||
|
|
|
|
Methods of Preparation
Suppositories can be extemporaneously prepared by one of three methods.
1. Hand Rolling is the oldest and simplest method of suppository preparation and may be used when only a few suppositories are to be prepared in a cocoa butter base. It has the advantage of avoiding the necessity of heating the cocoa butter. A plastic-like mass is prepared by triturating grated cocoa butter and active ingredients in a mortar. The mass is formed into a ball in the palm of the hands, then rolled into a uniform cylinder with a large spatula or small flat board on a pill tile. The cylinder is then cut into the appropriate number of pieces which are rolled on one end to produce a conical shape.
Effective hand rolling requires considerable practice and skill. The suppository “pipe” or cylinder tends to crack or hollow in the center, especially when the mass is insufficiently kneaded and softened.
2. Compression Molding is a method of preparing suppositories from a mixed mass of grated suppository base and medicaments which is forced into a special compression mold. The method requires that the capacity of the molds first be determined by compressing a small amount of the base into the dies and weighing the finished suppositories. When active ingredients are added, it is necessary to omit a portion of the suppository base, based on the density factors of the active ingredients.
3. Fusion Molding involves first melting the suppository base, and then dispersing or dissolving the drug in the melted base. The mixture is removed from the heat and poured into a suppository mold. When the mixture has congealed, the suppositories are removed from the mold. The fusion method can be used with all types of suppositories and must be used with most of them.
Suppositories are generally made from solid ingredients and drugs which are measured by weight. When they are mixed, melted, and poured into suppository mold cavities, they occupy a volume – the volume of the mold cavity. Since the components are measured by weight but compounded by volume, density calculations and mold calibrations are required to provide accurate doses.
When a drug is placed in a suppository base, it will displace an amount of base as a function of its density. If the drug has the same density as the base, it will displace an equivalent weight of the base. If the density of the drug is greater than that of the base, it will displace a proportionally smaller weight of the base. Density factors for common drugs in cocoa butter are available in standard reference texts. The density factor is used to determine how much of a base will be displaced by a drug. The relationship is:

For example, aspirin has a density factor in cocoa butter of 1.3 (see Remington’s). If a suppository is to contain 0.3 g of aspirin, it will replace 0.3 g ÷ 1.3 or 0.23 g of cocoa butter. If the blank suppository (suppository without the drug) weighed 2 g, then 2 g – 0.23 g or 1.77 g of cocoa butter will be needed for each suppository, and the suppository will weigh 1.77 g + 0.3 g = 2.07 g. So if a pharmacist was making 12 aspirin suppositories using cocoa butter as the base, he would weigh 1.77 g × 12 or 21.24 g of cocoa butter and 0.3 g × 12 or 3.6 g of aspirin.
Some example density factors of drugs in cocoa butter are shown in the table below (see Remington’s):
| Aspirin | 1.3 |
| Barbital | 1.2 |
| Bismuth salicylate | 4.5 |
| Chloral hydrate | 1.3 |
| Cocaine hydrochloride | 1.3 |
| Codeine phosphate | 1.1 |
| Diphenhydramine hydrochloride | 1.3 |
| Morphine hydrochloride | 1.6 |
| Phenobarbital | 1.2 |
| Zinc Oxide | 4.0 |
All About Suppository Molds and Packaging
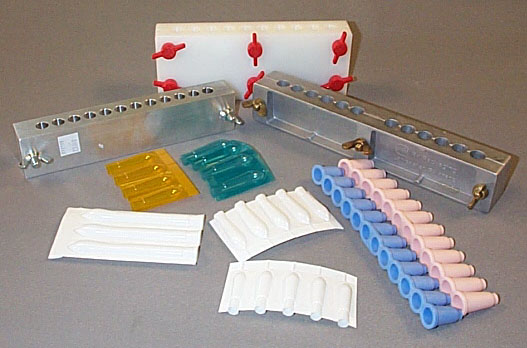
 Aluminum metal molds come in a variety of cavity sizes and with a variety of number of cavities per mold. Common sizes vary from 1 g to 2.5 g, and common number of cavities range from 6 cavities up to 100 cavities. The two halves of the mold are held together with either nuts or some molds have 1 centered screw.
Aluminum metal molds come in a variety of cavity sizes and with a variety of number of cavities per mold. Common sizes vary from 1 g to 2.5 g, and common number of cavities range from 6 cavities up to 100 cavities. The two halves of the mold are held together with either nuts or some molds have 1 centered screw.
 Plastic suppository shells come in long strips that can be torn into any number of cavities. The suppository mixture is poured directly into the shell up to a mark. These disposable molds do not need any lubrication regardless of the suppository mixture. When the mixture has hardened, the plastic mold is heat sealed. When a patient is ready to use a suppository, they select one shell and peel the sides of the shell off to obtain the suppository. One advantage of this type of mold is that if the suppository should melt, it will not run out of the mold. If the material can congeal again, it will retain the suppository shape. This type of mold is available in 1 g to 5 g sizes, and many different colors.
Plastic suppository shells come in long strips that can be torn into any number of cavities. The suppository mixture is poured directly into the shell up to a mark. These disposable molds do not need any lubrication regardless of the suppository mixture. When the mixture has hardened, the plastic mold is heat sealed. When a patient is ready to use a suppository, they select one shell and peel the sides of the shell off to obtain the suppository. One advantage of this type of mold is that if the suppository should melt, it will not run out of the mold. If the material can congeal again, it will retain the suppository shape. This type of mold is available in 1 g to 5 g sizes, and many different colors.
 There are also suppository molds made from flexible rubber. When the suppository mixture has congealed in these molds, the finished suppositories are “pushed” out of each cavity. If the prescription does not require all of the cavities in the strip, it can be trimmed with scissors. These flexible rubber molds are ideal if the suppositories need to be refrigerated (shells also are suitable for this purpose).
There are also suppository molds made from flexible rubber. When the suppository mixture has congealed in these molds, the finished suppositories are “pushed” out of each cavity. If the prescription does not require all of the cavities in the strip, it can be trimmed with scissors. These flexible rubber molds are ideal if the suppositories need to be refrigerated (shells also are suitable for this purpose).
 Very hard rubber molds are similar to the metal molds in that they have screws to hold the mold together. When the suppository mixture has congealed, the screws loosened, and the suppositories are removed. This photograph is a picture of an urethral mold. If you enlarge the image, you can see PEG suppositories in one side of the mold. Such thin suppositories require a great deal of investigation to get the desired consistency and strength. If the suppositories are too soft, it is very difficult to remove them from the mold. This particular casting had 70% PEG 3350 and 30% PEG 400.
Very hard rubber molds are similar to the metal molds in that they have screws to hold the mold together. When the suppository mixture has congealed, the screws loosened, and the suppositories are removed. This photograph is a picture of an urethral mold. If you enlarge the image, you can see PEG suppositories in one side of the mold. Such thin suppositories require a great deal of investigation to get the desired consistency and strength. If the suppositories are too soft, it is very difficult to remove them from the mold. This particular casting had 70% PEG 3350 and 30% PEG 400.
Pouring and Opening Suppository Molds
Molds should be filled only when they are at room temperature. A cold or frozen mold should never be used because it can cause fractures and fissures throughout the suppository. Each cavity should be filled slowly and carefully ensuring that no air bubbles are entrapped in the cavity. To prevent layering in the suppositories, the pouring process should not be stopped until all the cavities have been filled. Molds should be allowed to set at room temperature. Refrigeration should only be used if the suppository has not congealed after 30 to 40 minutes.
Aluminum molds usually require lubrication before use. Hard rubber molds may require lubrication. One way is to use a vegetable oil spray. Other lubricants include light mineral oil when water soluble bases are being used and glycerin or propylene glycol when oleaginous bases are being used. Whatever lubricant is used, only a light coating is needed. If too much lubricant is used, the excess will pool in the tip of the suppository cavity. Any excess lubricant should be wiped off with an absorbant tissue such as a Kimwipe.
When suppository mixtures and bases cool, they contract. Some mixtures and bases have very pronounced contractions (e.g., cocoa butter, PEG) while others have much smaller ones (e.g., glycerinated gelatin, MBK®). This contraction will produce a hole in the open end of the suppository. Such a hole is undesirable. If the suppository mixture is poured just immediately before it reaches its congealing temperature, the contraction will be minimized. It is also helpful to pour a small excess of the suppository mixture on top of the open end of the mold.
When filling a suppository mold, start pouring the melt at one end and pour continuously without stopping. Don’t go to the next cavity until the previous cavity is filled and a slight excess has been poured to overfill the cavity. Excess base can be removed once the suppositories have congealed by trimming the top of the mold with a warm stainless steel spatula.
Suppositories shells are generally poured using a back light to help visualize the mark on the shell. Some molds (depending on the size or type of the suppository) cannot be poured, but the mixture is added using a syringe.
Examples of each of these pouring (or filling techniques) are given.
When the suppository mixture has congealed, the excess mass is removed from the top surface of the mold and the mold is separated into the two halves. An efficient way to separate the mold is to remove the wing nuts or loosen the centered screw and place the mold so that the posts rest on the table top. Then apply a downward pressure only on the bottom half of the mold. A knife or spatula should not be used to pry the two halves apart. This will damage the matching mold faces which have been accurately machined to give a tight seal. (See Opening a Mold)
Suppository shells can be opened by peeling the halves apart if this type of shell is used. There are suppository shells that do not peel apart at the bottom but must be torn along its edge. These are very difficult to open, and should not be used.
 View a video demonstration on using a metal suppository mold
View a video demonstration on using a metal suppository mold
Suppositories that are not in a plastic shell mold or flexible rubber mold should be wrapped before they are dispensed. This will provide protection for the suppository and limit any oil staining that might occur from the materials contained in the suppository base.
 Once the suppositories are wrapped, they are generally placed in a special box that has dividers for each suppository.
Once the suppositories are wrapped, they are generally placed in a special box that has dividers for each suppository.
 Flexible rubber molds can be packaged with the suppository still in the mold. Generally the mold is placed in a special box.
Flexible rubber molds can be packaged with the suppository still in the mold. Generally the mold is placed in a special box.
Plastic shell molds must be heat-sealed. Heat sealing is generally a two step process. First the open end of the shell is “shrunk” with the aid of heat. A hair dryer (at highest hot setting) is capable of providing enough heat to shrink the plastic. There are laboratory type hot air “guns” that also can be used. The heat will cause the two sides of the opening to collapse together and begin to seal the opening. The second step is to use an electric sealer to completely seal the opening.
![]() View a video demonstration on filling and heat-sealing suppository shells
View a video demonstration on filling and heat-sealing suppository shells
Opening A Suppository Mold
Suppository shells can be opened by peeling apart the two tabs at the bottom of the shell.
When the Density Factor is Not Known
When bases other than cocoa butter are used, or when the density factor for a drug in cocoa butter is not known, then the density factor can be estimated by calculation or experimentally determined by the double casting technique.
The weight of the blank suppository is easily determined. A portion of the suppository base is melted, poured into the suppository mold and allowed to congeal. The suppositories are removed from the mold, and the total weight of the suppositories is determined. The average weight of the blank suppository is determined by dividing the total weight by the number of suppositories.
Estimation by Calculation
One method to determine the density factor of a drug in a base other than cocoa butter requires the use of the ratio of a blank suppository of the non-cocoa butter base to a blank suppository of the cocoa butter base. This information is generally obtained by calibrating the mold first with one base and then the other base.
As an example of the method, a mold was calibrated with the PEG base and the average blank suppository weighed 2.24 grams. The same mold was calibrated with cocoa butter and those blank suppositories weighed 1.87 grams on average. Therefore, the ratio of the two weights was:
![]()
If 200 mg of aspirin is to be incorporated into each PEG suppository, it is necessary to determine how much PEG base will be displaced by the aspirin. That displacement amount can be calculated as follows:
- density factor of aspirin in cocoa butter = 1.3 (from reference sources)
- density of PEG base relative to cocoa butter = 1.20 (the ratio obtained from the calibrations)
- 0.2 g of aspirin will displace
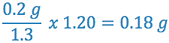 of PEG base
of PEG base
For each PEG suppository to be formulated, 0.2 g of aspirin and 2.06 g (2.24 g – 0.18 g = 2.06 g) of the PEG base will be needed.
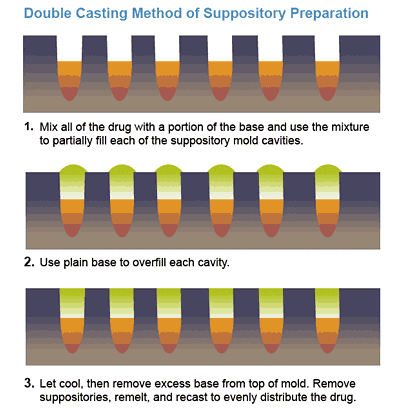
Double Casting Technique
The total quantity of drug is mixed with an amount of base which is inadequate to fill the number of cavities. The mixture is poured into the mold, partially filling each cavity, and the remaining portion of the cavities are filled with the melted blank base. The cooled suppositories are then removed, remelted, mixed, and recast to evenly distribute the active ingredient. By recording the necessary information, the pharmacist can determine the weight of base displaced by the drug and then calculate the density factor.
Note: a portion of the formula will be lost during this process, so you should always prepare for 2 extra suppositories to ensure that you have enough mixture for the desired number of suppositories.
![]() View a video demonstration on determining the density factor using the double casting technique
View a video demonstration on determining the density factor using the double casting technique
Sample calculation of density factor
Using a particular mold, the average weight of a plain cocoa butter suppository was found to be 2.0 g. Using the same mold, cocoa butter suppositories, each containing 300 mg of drug A, were found to weigh 2.1 g each. So,
-
- weight of suppository of cocoa butter = 2.0 g
-
- weight of drug in each medicated suppository = 0.3 g
-
- weight of suppository with drug and cocoa butter = 2.1 g
-
- weight of base in medicated suppository = 2.1 g – 0.3 g = 1.8 g
-
- weight of base displaced = 2.0 g – 1.8 g = 0.2 g
-
- Therefore, density factor of drug A = 0.3 g ÷ 0.2 g = 1.5
Now, knowing the density factor for the drug, the pharmacist can make calculations for a batch of suppositories. To prepare 10 suppositories:
-
- weight of drug A needed = 10 suppositories × 300 mg/suppository = 3000 mg = 3.0 g
-
- weight of base needed for plain suppositories = 10 suppositories × 2.0 g/suppository = 20.0 g
-
- weight of base displaced by 3 g drug A = 3.0 g ÷ 1.5 = 2.0 g
-
- weight of base needed for medicated suppositories = 20.0 g – 2.0 g = 18.0 g






