Powders & Granules
Upon completion of this exercise, you should be able to:
- List and describe a variety of types of powders and granules.
- Describe appropriate uses of pharmaceutical powders and granules.
- Compound and dispense divided and bulk powders and granule preparations.
The word “powder” refers to a chemical or mixture that is solid in physical state. In compounding, “powder” refers to a dosage formulation that is solid in physical state. But the formulation may be composed of only the active drug or may be a mixture of the active drug and other ingredients.
Powders offer some unique advantages:
- each dose can contain a different amount of active drug
- can be administered easily to infants and young children who cannot swallow tablets or capsules
- drug will have a rapid onset of action since disintegration is not required
- can be applied to many body cavities such as ears, nose, tooth socket, throat
- drugs tend to be most stable as a solid
- can be made into many different dosage formulations (capsules, tablets, powders for reconstitution, dusting powders, bulk powders, powders for inhalation, etc.)
Pharmaceutical powders are formulated to exist as fine particles. The powders are then smooth to the touch and nonirritating to the skin. Powders generally range from 0.1 to 10 micron in size. The size of the particles are often expressed as a number which corresponds to the mesh screen size of a sieve. The screen size indicates the number of openings in the mesh screen per inch. For example, a # 40 sieve has 40 openings per inch in the screen mesh. Particles that can sift through that mesh are said to be “40 mesh” size.
Below is a list of mesh sizes and the size of the mesh opening in millimeters (1/1000 of a meter) or microns (1/1,000,000) of a meter. There is a correlation between the size of the mesh opening and the particle size of the sifted powder. As the opening becomes smaller, so will the resulting particle size. Most of the particles of a sifted powder will have approximately the size as the mesh opening.
| Mesh Opening Size | ||
|---|---|---|
| Mesh Size Number | millimeters | microns |
| 2 | 9.52 | 9520 |
| 4 | 4.76 | 4760 |
| 8 | 2.38 | 2380 |
| 10 | 2.00 | 2000 |
| 20 | 0.84 | 840 |
| 30 | 0.59 | 590 |
| 40 | 0.42 | 420 |
| 50 | 0.297 | 297 |
| 60 | 0.250 | 250 |
| 70 | 0.210 | 210 |
| 80 | 0.177 | 177 |
| 100 | 0.149 | 149 |
| 120 | 0.125 | 125 |
| 200 | 0.074 | 74 |


The USP 24/NF19 uses descriptive terms to define powder fineness. The table below shows the correlation their classification.
| Description Term | Mesh Opening Size (microns) | Mesh Size Number |
|---|---|---|
| Very Coarse | > 1000 | 2 – 10 |
| Coarse | 355 -1000 | 20 – 40 |
| Moderately Coarse | 180 – 355 | 40 – 80 |
| Fine | 125 – 180 | 80 – 120 |
| Very Fine | 90 – 125 | 120 – 200 |
A good powder formulation has an uniform particle size distribution. If the particle size distribution is not uniform, the powder can segregate according to the different particle sizes which may result in inaccurate dosing or inconsistent performance. A uniform particle size distribution also insures an uniform dissolution rate if the powder is to dissolve, an uniform sedimentation rate if the powder is used in a suspension, and minimizes stratification when powders are stored or transported.
Reducing the particle size of a powder will result in an uniform distribution of particle sizes. The process of reducing the particle size is called comminution. In extemporaneous compounding, there are three methods of comminution:
- Trituration is the continuous rubbing or grinding of the powder in a mortar with a pestle. This method is used when working with hard, fracturable powders.
- Pulverization by Intervention is used with hard crystalline powders that do not crush or triturate easily, or gummy-type substances. The first step is to use an “intervening” solvent (such as alcohol or acetone) that will dissolve the compound. The dissolved powder is then mixed in a mortar or spread on an ointment slab to enhance the evaporation of the solvent. As the solvent evaporates, the powder will recrystallize out of solution as fine particles.
- Levigation reduces the particle size by triturating it in a mortar or spatulating it on an ointment slab or pad with a small amount of a liquid in which the solid is not soluble. The solvent should be somewhat viscous such as mineral oil or glycerin. This method is also used to reduce the particle size of insoluble materials when compounding ointments and suspensions.
Bulk Powders
Bulk powders are nonpotent and can be dosed with acceptable accuracy and safety using measuring devices such as the teaspoon, cup, or insufflator. This practically limits the use of orally administered bulk powders to antacids, dietary supplements, laxatives, and a few analgesics. Many bulk powders are used topically.
Dusting Powders
Dusting powders are fine medicinal (bulk) powders intended to be dusted on the skin by means of sifter-top containers. A single medicinal agent may be used as a dusting powder; however, a base is frequently used to apply a medicinal agent and to protect the skin from irritation and friction. Bentonite, kaolin, kieselguhr, magnesium carbonate, starch, and talc are used as inert bases for dusting powders. Powder bases absorb secretions and exert a drying effect, which relieves congestion and imparts a cooling sensation. All extemporaneous dusting powders should be passed through a 100-200 mesh sieve to ensure that they are grit free and will not further mechanically irritate traumatized areas.

Douche Powders
Douche powders are used to prepare solutions that cleanse the vagina. Most douche powders are used for their hygienic effects, but a few contain antibiotics.
Douche powders are prescribed as a matter of convenience for the patient, since a powder is more portable than a bulky solution. The formula is developed so that a teaspoonful or tablespoonful of powder dissolved in a specified volume of water provides the desired concentration. The pH usually ranges from 3.5 to 5 when the solution is prepared. Feminine bulb syringes or fountain syringes are used for vaginal irrigation. Since many of the ingredients are volatile (e.g., menthol, thymol, and volatile oils), douche powders should be packaged in glass jars with a wide mouth. Some commercial douche powders are available in metal foil packets, which contain the proper amount of powder for a single douche. Many douches are also available as prepared unit of use solutions in disposable applicators.
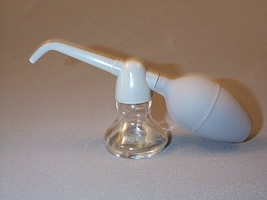
Insufflations
Insufflations are extremely fine powders to be introduced into body cavities. To administer an insufflation, the powder is placed in the insufflator, and when the bulb is squeezed, the air current carries the fine particles through the nozzle to the region for which the medication is intended. All extemporaneously compounded insufflations must be passed through a 100 mesh sieve. Pressurized packages provide an elegant approach to the administration of insufflations.
Powder Sprays
In contrast to dusting powders, powders dispensed under pressure will deliver targeted and uniform application at the desired site. Also, in an aerosol container medicated powders may be maintained in a sterile condition. The powder particles must be a definite size range to prevent clogging of the valve orifice and to provide uniformity of application. In general, powders that are to be packaged as powder sprays must not contain particles greater than 50 microns if they are to be sprayed successfully.
Divided Powders (Chartulae; Charts; Powder Papers)
Divided powders or charts are single doses of powdered medicinals individually wrapped in cellophane, metallic foil, or paper. The divided powder is a more accurate dosage form than bulk powder because the patient is not involved in measurement of the dose. Cellophane and foil-enclosed powders are better protected from the external environment until the time of administration than paper-enclosed powders.Divided powders are commercially available in foil, cellophane or paper packs.
Compounding with powders raises a concern about the compounder’s safety and the cleanliness of the compounding area. Typically, multiple dry powders are used in making a preparation, and the compounding process involves several activities. These activities aerosolize micronized particles that are only a few microns in diameters and not necessarily visible to the eye. There is a potential for the compounder to inhale these particles, for the powders to become cross-contaminated, and to fill the compounding area with ingredient particles.
 Powder containment units have been developed to minimize these potential risks. These hoods are low airflow laminar environments that bring negative pressure air through the face opening, sweep the air across the work surface, and then out through HEPA filtration. There are different kinds of powder containment hoods.
Powder containment units have been developed to minimize these potential risks. These hoods are low airflow laminar environments that bring negative pressure air through the face opening, sweep the air across the work surface, and then out through HEPA filtration. There are different kinds of powder containment hoods.
Another part of “containment thinking” involves wearing and not wearing gloves. It is best to wear gloves when securing, transferring, and weighing powder ingredients. But what if during those activities it is necessary to interact with a computer or some other device, or to retrieve weigh boats and utensils, etc. Should the compounder remove the gloves? They will be contaminated with powder particles. So containment should be planned for throughout the compounding procedure, and be specified in the Formulation Record.
Granules are particles ranging in size from about 4 to 10 mesh. Granules generally are made by first blending the powders together and then moistening the mixture to form a pasty mass. The mass is passed through a sieve and then dried in air or in an oven. They are prepared as a convenience for packaging, as a more stable product due to less surface exposure, and as a popular dosage form. Granulations are also used as intermediates in the preparation of capsules and tablets, since they flow more smoothly and predictably than do small powder particles.
The most popular compounded granulation is the effervescent powder (sometimes called effervescent salts). These granulations are popular due to their taste and psychological impression. When added to water, the granulation effervesces (“fizzes”) as carbon dioxide is liberated.
Preparation of Effervescent Granulation
It has been found that citric acid monohydrate and tartaric acid used in the ratio of 1:2, respectively, produces a powder with good effervescent properties. Citric acid monohydrate is not used alone because it results in a sticky mixture that will not easily granulate. Tartaric acid is not used alone because the granules are too friable and crumble. The amount of sodium bicarbonate to be used may be calculated from the reaction which occur when the granules come in contact with water. The reaction equation between citric monohydrate and sodium bicarbonate is given below:
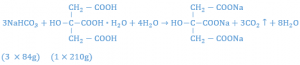
Setting up a proportion to determine the amount of sodium bicarbonate that will react with 1 gm of citric acid, one has:
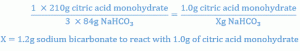
Similar calculations show that 2.24 gm of sodium bicarbonate react with 2 gm of tartaric acid.
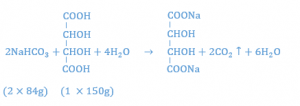

Thus, with the acids in a ratio of 1:2, it has been calculated that 3.44 g (1.2 g + 2.24 g) of sodium bicarbonate is necessary to react stoichiometrically with the 3 g of combined acids. To enhance the flavor, the amount of sodium bicarbonate may be reduced to 3.4 gm to allow for a small amount of unreacted acid to provide a tart taste.
Ibuprofen Effervescent Powder: scenario and formulation record