Aerosols, Foams, & Sprays
Upon completion of this exercise, you should be able to:
- Describe the advantages and limitations of different types of aerosol dosage forms.
- Describe the makeup of an aerosol formulation and system.
- Know the different parts and functions of an aerosol package.
- Describe how aerosol packages are filled with formulations.
- Explain how aerosol systems are clinically used.
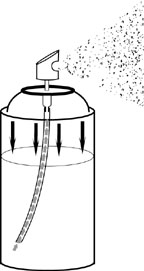
Aerosols are unique among the pharmaceutical dosage forms because they depend on the function of a container, its valve assembly, and propellants for the physical delivery of the ingredients. The aerosol container is referred to as a pressurized package. The pressure inside the package is created by the presence of one or more liquefied or gaseous propellants. When the valve is actuated, the pressure forces the contents of the package out through the opening in the valve. The physical form of the expelled contents is a function of the product formulation and the type of valve employed.
Aerosols used to provide an airborne mist are called space sprays and include room disinfectants and deodorizers. This group of aerosols produce particles that are usually less than 50 μm in size. This will ensure that the dispersed droplets or particles will remain airborne for a prolonged period of time. A one-second burst from a typical space spray will produce 120 million particles, of which a substantial number will remain airborne for an hour.
Aerosols intended to carry the active drug to a surface are called surface sprays or surface coating sprays. This class of sprays includes products such as deodorant sprays, hair sprays, perfume and cologne sprays, shaving lathers, paint sprays, and various household products such as spray starch, waxes, polishes, and cleaners.
Comparison of Space Sprays, Surface Sprays, and Foam Aerosols
| Property | Space Sprays | Surface Sprays | Foam Aerosols |
|---|---|---|---|
| Product Concentrate (%) | 2 – 20 | 20 – 75 | |
| Propellant (%) | 70 – 98 | 25 – 80 | 6 – 10 |
| Pressure (psig at 70°F) | 30 – 40 | 25 – 55 | 35 – 55 |
| Particles (μm) | <1 – 50 | 50 – 200 |
Pharmaceutical aerosols emit liquid or solid materials in a gaseous medium when they are actuated. The contents may be a fine mist, a course wet or dry spray, a steady stream, or a stable or fast-breaking foam. Pharmaceutical aerosols are intended to deliver active drugs for inhalation, nasal, buccal, and sublingual administration. Aerosols are also available for topical, rectal, and vaginal administration.
Some general advantages of pharmaceutical aerosols include:
- 1. Aerosols are easy to use. Medication is dispensed at the push of a button. No ancillary equipment is needed.
- 2. Aerosol application is a clean process which requires minimal patient cleanup after using the product.
- 3. A portion of medication may be easily withdrawn without contaminating the remaining material. If the product is sterile, sterility can be maintained throughout the product’s shelf life.
- 4. The active drug is protected from oxygen and moisture. The usual aerosol container is opaque, which also protects the drug from light.
- 5. By proper formulation and valve control, the physical form and the particle size of the emitted product may be controlled.
- 6. If the dosage must be regulated, a metered dose valve can be used which will control the accuracy of the administered dose.
Many pharmaceutical aerosols are used for oral (i.e., buccal and sublingual), nasal, or inhalation administration of vaccines, antiviral compounds, and hormones. These aerosols provide the advantage of a rapid onset of action and avoid the first pass effect and gastrointestinal tract degradation. In some cases, lower drug dosages can be used, which have the additional benefit of minimizing adverse reactions. Using these routes also provides a viable alternative for administration of drugs that exhibit erratic pharmacokinetics after oral or parenteral administration.
Other pharmaceutical aerosols are used for topical, vaginal, and rectal administration. Topical medications can be applied as a spray, foam, or semisolid in a uniform thin layer without having to touch or mechanically irritate the affected area. The use of an aerosol will also limit the potential for overuse of a product compared to lotions, creams, and ointments. For vaginal and rectal applications, aerosols can be delivered as an expanding foam to ensure a direct and extensive contact between the drug and the mucosa.
An aerosol formulation consists of two components: the product concentrate and the propellant. The product concentrate is the active drug combined with additional ingredients or co-solvents required to make a stable and efficacious product. The concentrate can be a solution, suspension, emulsion, semisolid, or powder. The propellant provides the force that expels the product concentrate from the container and additionally is responsible for the delivery of the formulation in the proper form (i.e., spray, foam, semisolid). When the propellant is a liquefied gas or a mixture of liquefied gases, it can also serve as the solvent or vehicle for the product concentrate. If the product characteristics are to change on dispensing, additional energy in the form of a mechanical breakup system may be required.
Propellants
A propellant is a chemical with a vapor pressure greater than atmospheric pressure at 40°C (105°F). Types of propellants commonly used in pharmaceutical aerosols include chlorofluorocarbons, hydrocarbons, hydrochlorofluorocarbons and hydrofluorocarbons, and compressed gases.
Chlorofluorocarbon (CFC) propellants
For many years, the chlorofluorocarbon (CFC) propellants P-11, P-12, and P-114 were used in aerosol products. Their use has been severely curtailed due to their role in depleting the ozone layer of the atmosphere. Since January 1996, worldwide production of these CFCs has been reduced to only the amount needed for aerosols used in the treatment of asthma and chronic obstructive pulmonary disease. Alternatives to P-12 (i.e., P-134a and P-227) have now been developed and are being incorporated in aerosol formulations. Currently, there are not alternatives for P-11 and P-114. Small amounts of P-11 are required in most aerosol suspensions to make a slurry of the active drug and other ingredients. It also is used to dissolve surfactants in some formulations.
P-11, P-12, and P-114 are the CFCs of choice for oral, nasal, and inhalation aerosols. These particular chlorofluorocarbon propellants are well accepted due to their relatively low toxicity and inflammability. The chlorofluorocarbons as a class are inert but P-11 is subject to hydrolysis and will form hydrochloric acid in the presence of water. The acid increases the corrosion of the container and may be irritating when applied to membranes. If water is present, P-12 or a mixture of P-12 and P-114 are used.
The CFCs are gases at room temperature that can be liquefied by cooling them below their boiling point or by compressing them at room temperature. For example, dichlorodifluoromethane (P-12) will form a liquid when cooled to -21.6°F or when compressed to 84.9 psia at 70°F (psia = pounds per square inch absolute). These liquefied gases also have a very large expansion ratio compared to the compressed gases (e.g., nitrogen, carbon dioxide). The usual expansion ratio for liquefied gases is about 1 to 240 which means that 1 mL of liquefied gas will occupy a volume of approximately 240 mL if allowed to vaporize. Compressed gases have an expansion ratio of about 3 to 10.
| Name | Formula | No. | V.P. @70°F (psia)a |
B.P. °F (1 atm) |
Liquid Density @70°F (g/mL) |
|---|---|---|---|---|---|
|
Trichloromonofluoromethane |
CCl3F |
11 |
13.4 |
74.7 |
1.485 |
|
Dichlorodifluoromethane |
CCl2F2 |
12 |
84.9 |
-21.6 |
1.325 |
|
Dichlorotetrafluoroethane |
CClF2ClF2 |
114 |
27.6 |
39.4 |
1.468 |
apsia (pounds per square inch absolute) = psig (pounds per square inch gauge + 14.7)
The numerical designations for fluorinated hydrocarbons propellants have been designed so the chemical structure of the compound can be determined from the number. The system consists of three digits.
- The digit at the extreme right refers to the number of fluorine atoms in the molecule.
- The second digit from the right represent one greater in the number of hydrogen atoms in the molecule.
- The third digit from the right is one less than the number of carbon atoms in the molecule; if this third digit is 0, it is omitted and a two-digit number is used.
- The capital letter “C” is used before a number to indicate the cyclic nature of a compound.
- The small letters following a number are used to indicate decreasing symmetry of isomeric compounds. The most symmetrical compound is given the designated number, and all other isomers are assigned a letter (i.e., a, b, etc.) in descending order of symmetry.
- The number of chlorine atoms in a molecule may be determined by subtracting the total number of hydrogen and fluorine atoms from the total number of atoms required to saturate the compound.
When a liquefied gas propellant or propellant mixture is sealed in an aerosol container with the product concentrate, an equilibrium is establish between the propellant which remains liquefied and a portion that vaporizes and occupies the upper portion of the container. The pressure at this equilibrium is referred to as the vapor pressure (expressed as psia) and is a characteristic of each propellant at a given temperature. Since the vapor pressure is exerted equally in all directions and is independent of the quantity of liquefied phase present, the pressure forces the liquid phase up the dip tube and out of the container when the valve is actuated. As the propellant reaches the air, it evaporates due to the drop in pressure and leaves the product concentrate as airborne liquid droplets or dry particles. As the liquid is removed from the container through the dip tube, the equilibrium between the propellant’s liquefied phase and vapor phase is rapidly re-established. Thus, the pressure within the container remains virtually constant and the product may be continuously released at an even rate and with the same propulsion.
In the case when there is no dip tube in the container, the container is used in the inverted position so that the liquid phase will be in direct contact with the valve. When the valve is actuated, the liquid phase is emitted and immediately reverts to the vapor phase in the atmosphere.
Hydrochlorofluorocarbons (HCFC) and Hydrofluorocarbons (HFC)
The hydrochlorofluorocarbons (HCFC) and hydrofluorocarbons (HFC) differ from CFCs in that they may not contain chlorine and have one or more hydrogen atoms. These compounds break down in the atmosphere at a faster rate than the CFCs resulting in a lower ozone depleting effect.
P-22, 142b, and 152a are used in topical pharmaceuticals. These three propellants have a greater miscibility with water and therefore are more useful as solvents compared to the other propellants. They are also slightly more flammable than the other propellants but this is not perceived as a disadvantage.
| Name | Formula | No. | V.P. @70°F (psia) |
B.P. °F (1 atm) |
Liquid Density @70°F (g/mL) |
|---|---|---|---|---|---|
|
Chlorodifluoromethane |
CHClF2 |
22 |
-135.7 |
-41.4 |
1.21 |
|
Trifluoromonofluoroethane |
CF3CH2F |
134a |
85.8 |
-15.0 |
1.21 |
|
Chlorodifluoroethane |
CH3CCIF2 |
142b |
43.8 |
14.4 |
1.12 |
|
Difluoroethane |
CH3CHF2 |
152a |
76.4 |
-12.5 |
0.91 |
|
Heptafluoropropane |
CF3CHFCF3 |
227 |
57.7 |
2.3 |
1.41 |
Hydrocarbons
The hydrocarbons are used in topical pharmaceutical aerosols because of their environmental acceptance and their low toxicity and nonreactivity. They are also useful in making three phase (two layer) aerosols because of their density being less than 1 and their immiscibility with water. The hydrocarbons remain on top of the aqueous layer and provide the force to push the contents out of the container. However, they are flammable and can explode. They contain no halogens and therefore hydrolysis does not occur making these good propellants for water based aerosols.
| Name | Formula | No. | V.P. @70°F (psia) |
B.P. °F (1 atm) |
Liquid Density @68°F (g/mL) |
|---|---|---|---|---|---|
|
Propane |
C3H8 |
A-108 |
124.7 |
-43.7 |
0.50 |
|
Isobutane |
C4H10 |
A-31 |
45.1 |
10.9 |
0.56 |
|
Butane |
C4H10 |
A-17 |
31.2 |
31.1 |
0.58 |
Propane, butane, and isobutane are the most commonly used hydrocarbons. They are used alone or as mixtures or mixed with other liquefied gases to obtain the desired vapor pressure, density, and degree of flammability. The flammability hazard has been substantially reduced by using mixtures of propellants and with the development of newer types of dispensing valves (i.e., valve with vapor tap).
Compressed Gases
Gases such as nitrogen, nitrous oxide, and carbon dioxide have been used as aerosol propellants for products dispensed as fine mists, foams, or semisolids. But due to their low expansion ratio, the sprays are fairly wet and the foams are not as stable as produced by liquefied gas propellants. However, using a compressed gas that is insoluble in the product concentrate (e.g., nitrogen) will emit the product concentrate in essentially the same form as it was placed in the container.
The pressure of the compressed gas contained in the headspace of the aerosol container forces the product concentrate out of the container. But unlike aerosols prepared with liquefied gas propellants, there is no propellant reservoir. So higher gas pressures are required in these aerosols and the pressure diminishes as the product is used. These gases have been used for the most part to dispense food products, dental creams, hair preparations, and ointments.
| Name | Formula | V.P. @70°F (psia) |
B.P. °F (1 atm) |
Gas Density @70°F (g/mL) |
|---|---|---|---|---|
|
Nitrogen |
N2 |
492 |
-320 |
0.97 |
|
Nitrous Oxide |
N2O |
735 |
-127 |
1.53 |
|
Carbon Dioxide |
CO2 |
852 |
-109 |
1.53 |
Product Concentrates
Solution aerosols are two phase systems consisting of the product concentrate in a propellant, a mixture of propellants, or a mixture of propellant and solvent. Solvents may also be added to the formulation to retard the evaporation of the propellant. Solution aerosols can be difficult to formulate because many propellant or propellant-solvent mixtures are nonpolar and are poor solvents for the product concentrate. Also, there is a limited number of solvents that can be used. Ethyl alcohol is the most commonly used solvent but propylene glycol, dipropylene glycol, ethyl acetate, hexylene glycol, and acetone have also been used.
Aerosol solutions have been used to make foot preparations, local anesthetics, spray-on protective films, anti-inflammatory preparations, and aerosols for oral and nasal applications. They contain 50 to 90% propellant for topical aerosols and up to 99.5% propellant for oral and nasal aerosols. As the percentage of propellant increases, so does the degree of dispersion and the finest of the spray. As the percentage of propellant decreases, the wetness of the spray will increase. The particle sizes of the sprays can vary from 5 to 10μm in inhalation aerosols and 50 to 100 μm for topical sprays.
Suspensions aerosols can be made when the product concentrate is insoluble in the propellant or mixture of propellant and solvent, or when a co-solvent is not desirable. Anti-asthmatic drugs, steroids, and antibiotics are delivered as suspension aerosols. When the valve is actuated, the suspension formulation is emitted as an aerosol and the propellant rapidly vaporizes and leaves a fine dispersion of the product concentrate.
Formulation considerations for suspension aerosols, not necessary with solution aerosols, include agglomeration, particle size growth, valve clogging, moisture content, and particle size of the dispersed aerosolized particles. Lubricants such as isopropyl myristate and light mineral oil, and surfactants such as sorbitan trioleate, oleic acid, and lecithin have been used to overcome the difficulties of particle size agglomeration and growth which are directly related to the clogging problems. The moisture content of the entire formulation should be kept below 200 to 300 ppm so all of the ingredients need to be the anhydrous form of the chemical or be capable of becoming anhydrous after a drying process. The particle size of the insoluble product concentrate ingredients should be in the 1 to 10 µm range for inhalation aerosols and between 40 to 50 µm for topical aerosols.
The product concentrate in an emulsion aerosol will consist of the active ingredient, aqueous and/or nonaqueous vehicles, and a surfactant. Depending on the components, the emitted product can be a stable foam (shaving cream type) or a quick breaking foam. A quick breaking foam creates a foam when emitted from the container but the foam collapses in a relatively short time. This type of foam is used to apply the product concentrate to a large area without having to manually rub or spread the product. Also, the active drug is more rapidly available because the foam quickly collapses.
Foams are produced when the product concentrate is dispersed throughout the propellant and the propellant is in the internal phase; i.e., the emulsion behaves like o/w emulsions. When the propellant is in the external phase (i.e., like a w/o emulsion), foams are not created but sprays or wet streams result. Stable foams are produced when surfactants are used that have limited solubility in both the organic and aqueous phases. Surfactants concentrate at the interface between the propellant and the aqueous phase forming a thin film referred to as the “lamella.” It is the specific composition of this lamella that dictates the structural strength and general characteristics of the foam. Thick and tightly layered lamellae produce very structured foams which are capable of supporting their own weight.
Surfactants used in emulsion aerosols have included fatty acids saponified with triethanolamine, anionic surfactants, and more recently nonionic surfactants such as the polyoxyethylene fatty esters, polyoxyethylene sorbitan esters, alkyl phenoxy ethanols, and alkanolamides. The nonionic surfactants are present fewer compatibility problems because they charge no electronic charge.
When liquefied gases (CFC, HCFC, HFC, hydrocarbons) are used as propellants, one of two systems can be formulated. The two phase system is the simplest system. Here the product concentrate is dissolved or dispersed in liquefied propellant and solvents creating a homogenous system. The propellants exist in both the liquefied phase and the vapor phase. When the aerosol valve is actuated, some liquefied propellant and solvent containing the product concentrate is emitted from the container. These aerosols are designed to produce a fine mist or wet spray by taking advantage of the large expansion of the propellant when it enters room temperature and atmospheric pressure. The two phase system is commonly used to formulate aerosols for inhalation or nasal application.
A three phase system (i.e., a heterogeneous system) is made up a layer of water immiscible liquid propellant, a layer of propellant immiscible liquid (usually water) which contains the product concentrate, and the vapor phase. This type of system is used when the formulation requires the presence of a liquid phase that is not propellant miscible. When the aerosol valve is actuated, the pressure of the vapor phase causes the liquid phase to rise in the dip tube and be expelled from the container. If the product is to maintain the liquefied gas reservoir, the dip tube must not extend beyond the aqueous phase. Sometimes it is desirable to have some liquefied propellant mixed with the aqueous phase to facilitate in the dispersion of the spray or to create a foam. In this case, the container should be shaken immediately prior to use.
If CFCs, HCFCs, and HFCs are used as the propellants, they will reside on the bottom of the container since their density is greater than water. The dip tube will then need to end somewhere in the middle of the container. If hydrocarbons are used as the propellants, they will reside on the aqueous layer since their density is less than water. In this case, the dip tube can be extended through the liquid propellant all the way down to the bottom of the container. Thus an important characteristic of any aerosol is the density of the propellant, propellants, or blend of propellants.
Foam aerosols are a three phase system in which the liquid propellant is emulsified with the product concentrate. When the valve is actuated, the emulsion is forced through the nozzle and the entrapped propellant reverts to the vapor phase and whips the emulsion into a foam when it reaches the atmosphere. To facilitate the formulation of a foam, some aerosols are shaken prior to use to disperse some of the propellant throughout the product concentrate. If a dip tube is present, the container is used while being held upright. If there is no dip tube, the container must be inverted prior to use.
Foam products operate at a pressure of about 40 to 50 psig at 70°F and contain about 4 to 7% propellant. Generally, a blend of propane and isobutane is used for foam aerosols. Contraceptive foam aerosols use A-31 as the propellant. Other foams use P-152a since it will produce a more stable foam and is less flammable than hydrocarbons. Other propellants that have been used include the compressed gases nitrous oxide and carbon dioxide. Typical products include whipped creams and toppings and several pharmaceutical and veterinary products.
Aerosols using compressed gases as the propellant operate essentially as a pressure package. The pressure of the gas forces the product concentrate out of the container in essentially the same form as it was placed in the container. Only the product concentrate is expelled; the compressed gas remains in the container occupying the headspace. The pressure drops in the container as the product concentrate is removed and the gas expands to occupy the newly vacated space. The pressure will continue to drop as the product concentrate is expelled. Therefore, the initial pressure in these containers is higher than used in liquefied gas aerosols and is usually 90 to 100 psig at 70°F. The amount of product left in the container after the pressure is exhausted varies with the viscosity of the product and loss of pressure due to gas seepage.
Depending on the nature of the formulation and the type of compressed gas used, the product may be dispensed as a semisolid (solid stream) foam or spray. Semisolid aerosols are used to dispense more viscous concentrates such as dental creams, hair dressings, ointments, creams, cosmetic creams, and foods.
In barrier pack systems, the propellant is physically separated from the product concentrate. The propellant pressure on the outside of the barrier serves only to push the contents from the container. In the piston type system, a polyethylene piston is fitted into the container. The product concentrate is placed into the upper portion of the container and a compressed gas or hydrocarbon gas is placed on the other side of the piston. The gas pushes against the piston and pushes the product concentrate out of the container when the valve is actuated. As the rises in the container, it scrapes against the side of the container which helps dispense most of the product concentrate.
This system is used to dispense cheese spreads, cake decorating icings, and ointments. Since these product concentrates are semisolid and viscous, they emit from the container as a lazy stream rather than a foam or spray. The piston type system is limited to viscous materials since liquids tend to pass around the edges of the piston into the gas compartment.
A collapsible plastic bag fitted into a container is another type of barrier pack system. In some systems, the bag is a thin walled aluminum pouch. The product concentrate is placed in the bag and the propellant surrounds the bag. The bag is accordion pleaded to prevent the gas from pinching it closed. These types of systems are used to dispense liquids as fine mists or streams, and semisolids as streams. They system can also be used for topical creams, ointments, or gels.
Gels that foam after being dispensed are placed in both the piston type and collapsible plastic bag type of system. The dispensed gel contains a low boiling liquid such as isopentane or pentane. The liquid will vaporized when the gel is placed in the warmth of the hands, producing the foaming gel.
The Valve Assembly
The effectiveness of a pharmaceutical aerosol depends on achieving the proper combination of product concentrate formulation, container, and valve assembly. The valve mechanism is the part of the product package through which the contents of the container are emitted. The valve must withstand the pressure required by the product concentrate and the container, be corrosive resistant, and must contribute to the form of the emitted product concentrate.
The primary purpose of the valve is to regulate the flow of product concentrate from the container. But the valve must also be multifunctional and regulate the amount of emitted material (metered valves), be capable of delivering the product concentrate in the desired form, and be easy to turn on and off. Among the materials used in the manufacture of the various valve parts are plastic, rubber, aluminum, and stainless steel.
The basic parts of a valve assembly can be described as:
- Actuator – the actuator is the button which the user presses to activate the valve assembly and provides an easy mechanism of turning the valve on and off. In some actuators, mechanical breakup devices are also included. It is the combination of the type and quantity of propellant used and the actuator design and dimensions that determine the physical form of the emitted product concentrate.
- Stem – the stem supports the actuator and delivers the formulation in the proper form to the chamber of the actuator.
- Gasket – the gasket, placed snugly with the stem, serves to prevent leakage of the formulation of the valve is in the closed position.
- Spring – the spring holds the gasket in place and also is the mechanism by which the actuator retracts when pressure is released thereby returning the valve to the closed position.
- Mounting Cup – the mounting cup which is attached to the aerosol container serves to hold the valve in place. Because the undersigned of the mounting cup is exposed to the formulation, it must receive the same consideration as the inner part of the container with respect to meeting criteria of compatibility. If necessary, it may be coated with an inert material to prevent an undesired interaction.
- Housing – the housing located directly below the mounting cup serves as the link between the dip tube and the stem and actuator. With the stem, its orifice helps to determine the delivery rate and the form in which the product is emitted.
- Dip Tube – the dip tube which extends from the housing down into the product concentrate serves to bring the formulation from the container to the valve. The viscosity of the product and its intended delivery to rate dictate the inner dimensions of the dip tube and housing for a particular product.
Spray valves are used to obtain fine to coarse wet sprays. Depending on the formulation and the design of the valve and actuator, the particle size of the emitted spray can be varied. The spray is produced as an aerosol solution passes through a series of small orifices which open into chambers that allow the product concentrate to expand into the proper particle size.
Vapor tap valves are used with powder aerosols, water based aerosols, aerosols containing suspended materials, and other agents that would tend to clog a standard valve. This valve is basically a standard valve except that a small hole has been placed into the valve housing. This allows vaporized propellant to be emitted along with the product concentrate and produces a spray with greater dispersion. These valves are used with aqueous and hydroalcoholic product concentrates and hydrocarbon propellants.
Foam valves have only one orifice that leads to a single expansion chamber. The expansion chamber also serves as the delivery nozzle or applicator. The chamber is the appropriate volume to allow the product concentrate to expand into a ball of foam. Foam valves are used for viscous product concentrates such as creams and ointments because of the large orifice and chamber. Foam valves also are used to dispense rectal and vaginal foams. If the size of the orifice and expansion chamber are appropriately reduced, a product concentrate that would produce a foam will be emitted as a solid stream. In this case, the ball of foam begins to develop where the stream impinges on a surface.
Metered dose inhaler (MDI) valves (metering valves) are used to accurately deliver a dose of medication. Metered valves are used for all oral, inhalation, and nasal aerosols. The metered valves reproducibly deliver an amount of product concentrate accurately from the same package and also allow for the same accuracy between different packages.
The amount of material emitted is regulated by an auxiliary valve chamber of fixed capacity and dimensions. This metering chamber volume can be varied so that about 25 to 150 mL of product concentrate is delivered per actuation. Access in and out of the metering chamber is controlled by a dual valve mechanism. When the actuator is closed, a seal blocks emission from the chamber to the atmosphere. However, the chamber is open to the contents of the container and it is filled. When the actuator is depressed, the seals reverse function; the chamber becomes open to the atmosphere and releases its contents and at the same time becomes sealed from the contents of the container. When the actuator is again closed, the system prepares for the next dose.
Two basic types of metering valves are available; one for inverted use and the other for upright use. Generally the valves for upright use are used with solution type aerosols and contain a thin capillary dip tube. Suspension or dispersion aerosols use the valve intended for inverted use that does not contain a dip tube.
In general, valves should retain the material in the metering chamber for fairly long periods. However, it is possible for the material in the chamber to slowly return back to the container. The degree to which this occurs depends on the construction of the valve and length of time between actuations of the valves. Some valves have been fitted with a “drain tank” to overcome this problem.
Containers
Aerosol containers are generally made of glass, metals (e.g., tin plated steel, aluminum, and stainless steel), and plastics. The selection of the container for a particular aerosol product is based on its adaptability to production methods, compatibility with the formulation, ability to sustain the pressure necessary for the product, the design and aesthetic appeal, and the cost.
| Container Material |
Maximum Pressure (psig) |
Temperature (oF) |
|---|---|---|
| Tin-plated steel |
180
|
130
|
| Uncoated glass |
< 18
|
70
|
| Coated glass |
< 25
|
70
|
| Aluminum |
180
|
130
|
| Stainless Steel |
180
|
130
|
| Plastic |
< 25
|
70
|
Glass containers would be the preferred container for most aerosols. Glass presents fewer problems with respect to chemical compatibility with the formulation compared to metal containers and is not subject to corrosion. Glass is also more adaptive to design creativity and allows the user to view the level of contents in the container.
However, glass containers must be precisely engineered to provide the maximum pressure safety and impact resistance. Therefore, glass containers are used in products that have lower pressures and lower percentages of propellants. When the pressure is below 25 psig and less than 50% propellant is used, coated glass containers are considered safe.
To increase the resistance to breakage, plastic coatings are commonly applied to the outer surface of glass containers. These plastic coatings serve many purposes: 1) prevent the glass from shattering into fragments if broken; 2) absorb shock from the crimping operation during production thus decreasing the danger of breakage around the neck; 3) protect the contents from ultraviolet light; 4) act as a means of identification since the coatings are available in various colors.
Glass containers range in size from 15 to 30 mL and are used primarily with solution aerosols. Glass containers are generally not used with suspension aerosols because the visibility of the suspended particles presents an aesthetic problem. All commercially available containers have a 20 mm neck finish which adapts easily to metered valves.
Tin-plated steel containers are light weight and relatively inexpensive. For some products the tin provides all the necessary protection. However when required, special protective coatings are applied to the tin sheets prior to fabrication so that the inside of the container will be protected from corrosion and interaction between the tin and the formulation. The coating usually is an oleoresin, phenolic, vinyl, or epoxy coating. The tin plated steel containers are used in topical aerosols.
Aluminum is used in most MDIs and many topical aerosols. This material is extremely light weight and is less reactive than other metals. Aluminum containers can coated with epoxy, vinyl, or phenolic resins to decrease the interaction between the aluminum and the formulation. The aluminum can also be anodized to form a stable coating of aluminum oxide. Most aluminum containers are manufactured by an impact extrusion process that make them seamless. Therefore, they have a greater safety against leakage, incompatibility, and corrosion.
Aluminum containers are made with a 20 mm neck finish that adapts to the metered valves. For special purposes and applications, containers are also available that have neck finishes ranging from 15 to 20 mm. The container themselves available in sizes ranging from 10 mL to over 1,000 mL.
Stainless steel is used when the container must be chemically resistant to the product concentrate. The main limitation of these containers is their high cost.
Plastic containers have had limited success because of their inherent permeability problems to the vapor phase inside the container. Also, some drug-plastic interactions have limited the efficacy of the product.
Two methods are used to manufacture aerosols: the cold fill process and the pressure fill process. The cold fill process takes advantage of the property that some ingredients will liquefy when cooled, and the pressure fill process uses the property that some ingredients will liquefy when placed under pressure.
In the cold fill process, both the product concentrate and the propellant must be cooled to temperatures between 30°C to 60°C where they will remain liquefied. The cooling system may be a mixture of dry ice and acetone or an elaborate refrigeration system. The chilled product concentrate is quantitatively added to the equally cold aerosol container and then the liquefied gas is added. The heavy vapors of the cold liquid propellant will generally displace the air present in the container. When filling is complete, the valve assembly is inserted into the container and crimped into place. The container is then passed through a water bath of about 55°C to check for leaks or distortion in the container.
Aqueous solutions cannot be filled by this process since the water will turn to ice in the low temperatures. For nonaqueous systems, some moisture usually appears in the final product due to the condensation of atmospheric moisture within the cold containers.
Pressure filling is carried out essentially at room temperature. The product concentrate is placed in the container, the valve assembly is inserted and crimped into place, and then the liquefied gas, under pressure, is added through the valve. The entrapped air in the package might be ignored if it does not interfere with the stability of the product, or it may be evacuated prior to or during filling. After the filling operation is complete, the valve is tested for proper function. This spray testing also rids the dip tube of pure propellant prior to consumer use. Pressure filling is used for most pharmaceutical aerosols. It has the advantage that there is less danger of moisture contamination of the product and also less propellant is lost in the process.
Aerosols are used to deliver active drugs to the pulmonary airways, the nasal passages, or the oral cavity. They are also used to administer drugs topically and into body cavities such the vagina and rectum. Pulmonary, nasal, and oral administration is intended to achieve either local or systemic therapeutic effect, while topical, vaginal, and rectal administration is only intended for local effect.
Inhalation therapy (i.e., drug delivery to the pulmonary airways and nasal passages) was once accomplished using nebulizers or atomizers that were cumbersome to use and restricted to institutional or home use. The development of the metered dose inhaler in the mid 1950s provided the convenience of portability with the accuracy of dosing.
Successful inhalation therapy requires that the formulation emit droplets or particles that are the optimum size. Large particles (about 20 µm) deposit in the back of the mouth and throat and are eventually swallowed rather than inhaled. Particles in the 1 to 10 µm range will reach the bronchioles. Very small particles (0.6 µm) penetrate to the alveolar sacs but have limited retention since a large fraction of the particles are exhaled in the breath. The most therapeutically effective particle size range appears to be between 3 and 6 µm Therefore, it is important that the aerosol system produce most of its particles between approximately 1 and 10 µm.
MDIs are the most commonly used product for inhalation therapy and is also one of the most difficult dosage forms to administer properly. One of the most critical maneuvers during administration is to coordinate the actuation of the aerosol with the patient’s inspiration. The mouthpiece adapter on the aerosol package has been repeatedly modified since the mid 1970s in an attempt to help patients receive the correct dosage when this coordination is not performed correctly. Larger adapters (sometimes called tube spacers) permit the propellant to completely evaporate before the aerosol reaches the patient. This results in a reduced particle size and velocity. The reduced particle size improves the depth to which the drug will penetration into the lungs and the lower velocity decreases the amount of drug that will impact on the back of the throat. The biggest disadvantages of these larger adapters are the cost, difficulty to clean, and inconvenience to use because of their size.
Nasal aerosols deliver the drug directly to the nasal mucosa. The most common nasal aerosols contain steroids used to treat nasal congestion, sneezing, and running nose associated with hay fever, allergies, and rhinitis. Such products use steroids such as beclomethasone dipropionate, triamcinolone acetonide, dexamethasone sodium phosphate, and budesonide. Aerosols used to deliver drugs to the oral cavity generally administer the product sublingually. One such product is a sublingual nitroglycerin formulation that is sprayed under the tongue and delivers 0.4 mg of nitroglycerin per actuation.
Topical aerosol formulations are available for local anesthetics, antiseptics, germicides, first aid preparations, and spray on protective films. These aerosol deliver particles that are larger and more course than the inhalation aerosols. Topical aerosols deliver the active drug in the form of a powder, a wet spray, a stream of liquid, or an ointment like product.
Vaginal and rectal foams are available that contain estrogens, contraceptives, and anti-inflammation agents. These products are packaged in containers that have an application device which is filled with foam when the valve is actuated and then the device is placed in the vagina or rectum and the foam is instilled with the device plunger.
Gas molecules travel in random paths and collide with one another and the walls of their container. These collisions exert a pressure per unit area and also cause the gases to occupy a volume. Both the pressure and volume are affected by temperature. The interrelationships between these three variables were formulated by Boyle, Charles, and Gay-Lussac, and can be applied to pharmaceutical aerosols.
Boyle’s Law states that:
![]() when temperature does not change
when temperature does not change
and:
![]()
where:
![]()
![]()
![]()
Charles’s or Gay-Lussac’s Law states that:
![]() when pressure does not change
when pressure does not change
and:
![]()
where:
![]()
If two sets of conditions are being considered, these equations can be combined to obtain the relationship:
![]()
where the subscripts 1 and 2 refer to two different conditions. Although the P, V, and T of each condition may be different, the ratio ![]() is constant and can be mathematically expressed as:
is constant and can be mathematically expressed as:
![]()
where:
![]()
This equation is derived considering only 1 mole (i.e., one gram molecular weight) of ideal gas. If n moles of gas were to be considered, it becomes:
![]()
which is known as the General Ideal Gas Law. R is the molar gas constant and is used with many different units depending on the mathematical application: 8.314 J/K/mole, 0.08205 L atm/K/mole, and 1.987 cal/K/mole.
The relevance of the gas laws to pharmaceutical aerosols can be seen in the following examples.
Example 1: What is the weight of nitrogen in an 8 fluid ounces aerosol container filled with 6 fluid ounces of viscous ointment? The container is pressurized to 90 psig and the temperature is 25°C.
Determine the following values:
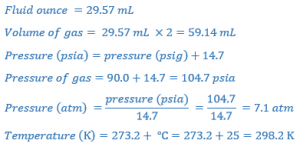
From a rearrangement of the General Ideal Gas Law:
![]()
Converting the number of moles to a weight can be done using the atomic weight of nitrogen (i.e., 14) and its valence, which is 2:
![]()
Example 2: If 3 fluid ounces of the aerosol are dispensed, what is the resulting pressure in the container?
The Ideal Gas Law can be rearranged, noting that T1 = T2 (i.e., temperature has not changed), by setting V1 and P1 to the initial values for the container (i.e., 2 fluid ounces of gases), and solving for V2 and P2 after the container as emitted 3 fluid ounces (i.e., there are now 5 fluid ounces of gas):
![]()
![]()
![]()
Example: What is the pressure remaining in the container when all of the product has been dispensed?
The equation from the previous example can still be used. However, the gas now occupies 8 fluid ounces.
![]()
![]()
A liquefied gas propellant can be considered as a solution. Molecules of the solution will have escape tendencies that will create a vapor pressure above the solution. According to Raoult’s Law, if a solute is added to a solvent, the solvent vapor pressure will be decreased proportional to the mole fraction of the solute added. If the added solute has an appreciable vapor pressure itself, its vapor pressure will also be decreased as the result of its dilution in the solvent. The total vapor pressure of a mixture of propellants can be determined as the sum of the partial pressures of each component (Dalton’s Law). The partial pressure of a component can be determined as the mole fraction of the component multiplied by the vapor pressure of the pure compound. If propellant a and b are mixed, the partial pressures can be calculated as:
![]()
![]()
where:

The total vapor pressure of the aerosol would be the sum of the two partial pressures calculated above:
![]()
Example: What is the vapor pressure of a mixture of propellants 11 and 12 in a 70 g to 30 g ratio?
| Chemical Name | Chemical Formula | Numerical Designation | Vapor Pressure 70°F (psia)a | Molecular Weight |
|---|---|---|---|---|
| Trichloromonofluoromethane | CCl3F | 11 | 13.4 | 137.38 |
| Dichlorodifluoromethane | CCl2F2 | 12 | 84.9 | 120.93 |
apsia (pounds per square inch absolute) = psig (pounds per square inch gauge + 14.7)
Determine the mole fractions of each propellant.
![]()
![]()
Determine the partial pressure for each propellant.
![]()
![]()
The total vapor pressure for the propellant mixture will be:
![]()