The Absorption Spectrum
If we monitor a beam of light shining through a sample containing a substance
that can absorb one of the beam's wavelengths, we can obtain a plot of the amount
of light absorbed versus the wavelength, as shown in Figure 1a. This plot is
known as an absorption spectrum, and shows which particular wavelengths
of light a chemical species can absorb. The energy associated with the absorbed
wavelength corresponds to the energy difference between the electronic
levels involved in the excitation of the electrons in the absorber. In solution,
these energy levels are modified by the properties of the solvent. Subsequently,
they can have a number of different energy values. Thus, when we repeat the
experiment with a molecule or ion in solution, many different wavelengths adjacent
to the main transition wavelength are also absorbed. This is shown in Figure
1b.
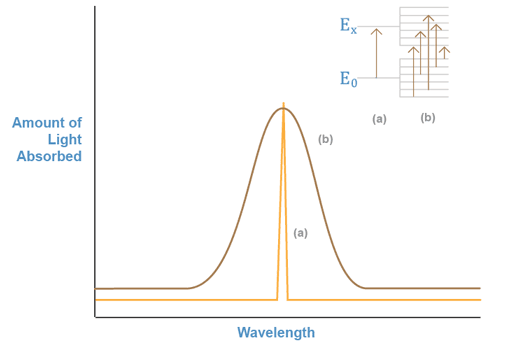
Figure 1: Absorbed Light vs. Wavelength
The absorption spectrum for a substance can be used to identify the
presence of that substance, since every chemical species has a specific
set of energy levels that it can absorb, depending on its unique electronic
configuration. Unfortunately, the levels of these species in solution
are modified so greatly that many different substances assume similar
levels. Thus, the absorption spectra for many compounds and ions look the
same.