Quantitative Measurements
A primary use of absorption spectroscopy lies in its applicability to
quantitative measurements. This is a function of how much light is absorbed,
and how that relates to the amount of the absorber present in the sample.
The following derivation presents the basics of this relationship.
When we shine a light beam of a certain wavelength (l) and initial intensity (I 0 ) through an absorbing sample contained in a spectrophotometer cell,
the intensity of the light beam transmitted through the sample (I t )
is dependent on three factors (see Figure 2). The first factor is whether
the sample will absorb light at that wavelength. The second is the amount
of sample which the light must pass through or, the cell width (b).
The third factor is the concentration of the absorbing species in
the sample solution (C). You can see through a test tube containing micromolar
concentrations of the same species. The fraction of light transmitted,
or transmittance (T), is defined as the following:
 (1)
(1)
and,
 (2)
(2)
where,

The transmittance of the sample varies logarithmically with the cell
width and the concentration of the absorbing species in the following way:
 (3)
(3)
The proportionality constant depends on the chemical nature of the individual
absorber, the wavelength at which the measurements are being made, and
the units of b and C. In visible absorption spectroscopy, b is normally
measured in centimeters. If C is also measured in mol/L (molar concentration,
M), the proportionality constant is defined as the molar absorptivity
(ε), which has units of l/M-cm. If C is measured in any other units (e.g.,
g/l), the constant is simply called the absorptivity (a), whose
units will depend on C. Under normal operating conditions, ε or a is determined
experimentally by measuring a standard of known concentration.
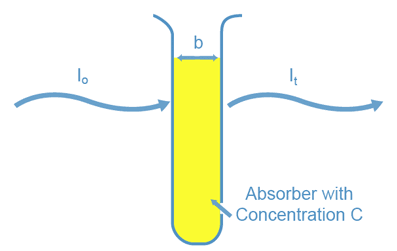
Figure 2: Factors that Determine Transmitted Light Intensity