Intravenous
 Several
sites on the body are used to intravenously administer drugs: the veins of the
antecubital area (in front of the elbow), the back of the hand, and some of
the larger veins in the foot. On some occasions, a vein must be exposed by a
surgical cut down.
Several
sites on the body are used to intravenously administer drugs: the veins of the
antecubital area (in front of the elbow), the back of the hand, and some of
the larger veins in the foot. On some occasions, a vein must be exposed by a
surgical cut down.
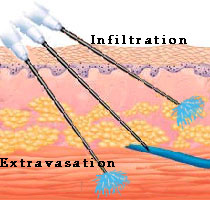 Intravenous
administration will provide the most rapid onset of action of any parenteral
route because there is no barrier to absorption. The drug is completely available
to the body. Drugs that are too irritating for intramuscular or subcutaneous
administration (e.g., chemotherapy agents) can be given by this route. Many
different types of catheters (an indwelling soft tube) or needles are used to
administer intravenous formulations. Placement of these devices is crucial to
avoid problems of extravasation or infiltration.
Intravenous
administration will provide the most rapid onset of action of any parenteral
route because there is no barrier to absorption. The drug is completely available
to the body. Drugs that are too irritating for intramuscular or subcutaneous
administration (e.g., chemotherapy agents) can be given by this route. Many
different types of catheters (an indwelling soft tube) or needles are used to
administer intravenous formulations. Placement of these devices is crucial to
avoid problems of extravasation or infiltration.
There are several complications that can occur from intravenous
administration.
- Thrombosis formation can results from
many factors: extremes in solution pH, particulate material, irritant properties
of the
 drug, needle or catheter trauma, and selection of too small a vein for the
volume of solution injected.
drug, needle or catheter trauma, and selection of too small a vein for the
volume of solution injected.
- Phlebitis, or inflammation of the vein,
can be caused by the same factors that cause thrombosis.
- Air emboli occur when air is introduced
into the vein. The human body is not harmed by small amounts of air, but a
good practice is to purge all air bubbles from the formulation and administration
sets before use.
- Particulate material is generally small
pieces of glass that chip from the formulation vial or rubber that comes from
the rubber closure on injection vials. Although great care is taken to elimination
the presence of particulate material, a final filter in the administration
line just before entering the venous system is a typical precaution.
Solutions are the most commonly administered intravenous formulation. They
are usually aqueous but may also have glycols, alcohols, or other nonaqueous
solvents in them. Injectable suspensions are difficult to formulate because
they must possess syringeability and injectability. Syringeability refers to
the ease at which the suspension can be withdrawn from a container into a syringe.
It has been demonstrated that parenteral injections containing particles
>25µm3 in size and having aspect ratios of >3.1 frequently cause syringe needle blockage.
Injectability refers to the properties of the suspension while being injected,
properties such as flow evenness, freedom from clogging, etc. Intravenously
administered emulsions are heterogeneous formulations that contain both aqueous
and non-aqueous components. Fat emulsions and total parenteral nutrition (TPN)
emulsions are used to provide triglycerides, fatty acids, and calories for patients
who cannot absorb them from the gastrointestinal tract.